The morphogen Sonic Hedgehog (Shh) plays a critical role in the development of different tissues, both in invertebrates and vertebrates. Shh mediates its action via a receptor complex associating two transmembrane proteins: Patched1 (Ptch1) and Smoothened (Smo). The repression exerted by Ptch1 on Smo is relieved when Shh, the ligand, binds to Ptch1, which leads to a complex signaling cascade involving the transcription factors of the Gli family and finally, to the activation of targeted genes including Ptch1 and Gli themselves.
In the mammalian central nervous system, Shh is essential in the patterning of the developing neural tube system, limbs, axial skeleton and other derivatives of the somites during early embriogenesis. Many types of neuronal and nonneuronal cells differentiate in response to Shh, that shows a spatially restricted pattern of cellular expression. The distinct fate assumed by cells in response to Shh is dependent upon their position with respect to both the dorso-ventral and anterior-posterior axes of the Shh expressing cells in a concentration and time-dependent manner. At later embryonic stages, Shh begins to be expressed in other brain regions, including the hippocampal dentate gyrus (DG). This morphogen is also a key factor for the formation of the neural germinal niches located in the lateral ventricles and the subgranular zone of the DG, the two main locations where neurogenesis in the brain of adult mammals occurs. In the rodent brain, thousands of new granule neurons are generated daily in the DG, with the primary function of conferring an additional layer of plasticity to the brain. Adult hippocampal neurogenesis adds particular functionality to the mammalian hippocampus and presumably is involved in cognitive functions. Failing or altered neurogenesis has been associated with a number of neuropsychiatric diseases including anxiety and depression (Winner et al., 2011).
Although neurogenesis continues throughout life, its rate declines with increasing age. The maturation of granule cells generally mimics the neuronal maturation process observed in development, although at a slower time scale. Adult hippocampal neurogenesis originates from a population of precursor cells with glial properties, known as neural stem cells (NSCs). Survival and acquisition of a neuronal phenotype by NSCs are dependent on the existence of a complete environment, the “neurogenic niche”. Niches are defined as microenvironments that anatomically house stem cells and functionally control their development in vivo, supporting survival, maturation and function by the production of neurotrophins and other regulators of proliferation and differentiation. In the adult brain neurogenic niches exert important physiological homeostatic role, including production of new neurons in the hippocampus that play essential role in memory function, as well as in pathological condition, for instance after injury, when they might contribute to the formation of new cells upon central nervous system tissue damage. The prevailing model of adult hippocampal neurogenesis suggests that adult NSCs, residing in the subgranular zone, are maintained in a largely quiescent state. Upon activation by niche-derived and intrinsic signals, NSCs undergo proliferation to generate proliferating transit amplifying progenitors that enlarge the pool of neurogenic cells, giving rise to immature neurons. These cells progress through neuronal differentiation to granule cell neurons that integrate into functional neuronal circuits (Kempermann and Gage, 2000). The neurogenic niche is structured by a complex organization of different cell types, including the NSCs-neuron lineage, glial cells and vascular cells. This system provides a great variety of signals, including Shh, that orchestrate the control of adult neurogenesis, regulating the balance between self-renewal and differentiation of NSCs.
While the role of Shh pathway in central nervous system development and tumorigenesis has been extensively established, its role as a regulator of adult hippocampal neurogenesis is still largely uncovered, even though it has been shown to regulate proliferation of adult hippocampal NSCs (Lai et al., 2003). We investigated the in vivo effect of the Shh pathway activation on both hippocampal morphogenesis and neurogenesis, making use of mice with germline inactivation of one copy of the Ptch1 gene (Hahn et al., 1998), as Ptch1 homozygous inactivation causes death by embryonic day 9.5 because of the defects in the developing nervous and cardiovascular system. Our investigations highlighted for the first time, the role of Shh in shaping the DG, since Shh signaling activation in Ptch1+/– mice causes patterning defects, detectable as elongation and thinning of the DG blades, already visible at 10 days and persisting at 2 and 8 months of age (Antonelli et al., 2018). Moreover, to detect eventual Shh-dependent modification in the cellular composition of the subgranular zone of the DG, we applied criteria based on a combination of morphological cellular features and immunohistochemical labelling with stage-specific adult neurogenesis markers (Figure 1A). We detect defect in 2 months old Ptch1+/– mice (Antonelli et al., 2018) that progressively increased in severity manifesting as a significant depletion of radiali glia-like (RGL) cells (glial fibrillary acidic protein (GFAP)+) and accumulation of transient amplifying progenitor cells (Sex determining region Y (SRY) box 2 (Sox2)+), as well as a decrease in the number of immature (doublecortin (DCX)+) and mature (NeuN+) granule neurons in 8 months old Ptch1+/– mice (Figure 1B). Our findings demonstrate that constitutive activation of Shh pathway causes progressive defects in the dynamic transition among neural stages in the DG, including self-renewal, proliferation and differentiation, pointing to a complex and multi-faced role for Shh in the hippocampus going from DG shaping at embryonic/neonatal age to the control of progression of NSCs into neurons during adulthood.
Figure 1.
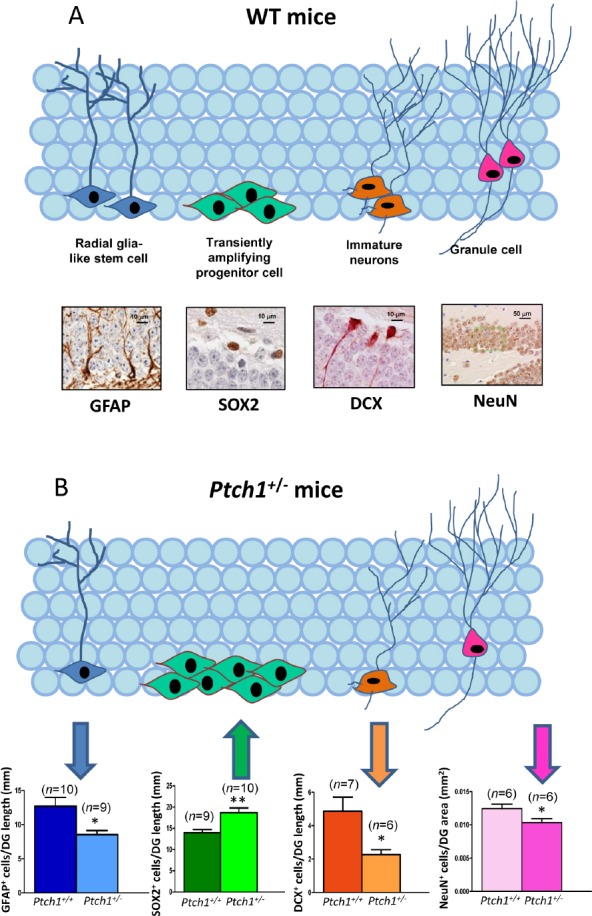
Sonic Hedgehog (Shh) pathway deregulation alters the cell stage composition of dentate gyrus (DG) in Ptch1+/– mice.
(A) Schematic representation of adult neurogenesis in the hippocampal DG and relative stage specific markers with representative immunostaining images and (B) quantification for glial fibrillary acidic protein (GFAP), Sex determining region Y (SRY) box 2 (Sox2), doublecortin (DCX), and mature granule (NeuN) at 8 months of age. Upward and downward arrows respectively represent an increase or deacrease in the number of labelled cells in Ptch1+/– compared to wild-type (WT) mice. Images, 20× magnification, scale bar: 50 µm; 100× magnification, scale bar: 10 µm. The number of mice used per test is indicated in the graphs (n). Data are reported as mean ± SEM. *P < 0.05, **P < 0.01 for comparison with controls (Student’s t-test). Modified from Antonelli et al. (2018). Ptch1: Patched1.
Dynamics and regulation of gene transcription in maturing neurons are finely controlled by the neurogenic niche. Similar to their embryonic counterparts, adult NSCs activate intrinsic programs based on the sequential activation of transcriptional factors that regulate neurogenesis in the context of the mature nervous system environment. The transcriptional network governing the production of new neurons in the adult subgranular zone is relatively unknown compared with embryonic neurogenesis and the Ptch1 mouse model can contribute to a better understanding of the molecular mechanisms involved in the adult neurogenesis control. Given that Shh-dependent deregulation of neurogenesis in the DG is progressive we thought to investigate the underlying molecular mechanisms by analyzing possible differences between Ptch1+/– and wild-type mice in the expression profiles of 84 genes with established roles in neurogenesis at 8 months, when the defects are more severe. At the molecular level, DG from Ptch1+/− mice exhibited deregulation of several downstream components of Notch signaling pathway that plays a pivotal role in NSC maintenance and in the regulation of glial versus neuronal identity. Many evidences demonstrate that Shh signaling can be modulated by the Notch pathway (Kong et al., 2015). Instead, we detected perturbations of key components of the Notch signaling pathway (Notch2; Hey1/Hey2; Neurog1/Neurog2 and Pax6) in Ptch1+/– mice, demonstrating that Shh signaling may in turn influence Notch pathway activity and, therefore, suggesting a more complex interplay between these pathways. The multifaced role of Shh in the regulation of adult neurogenesis also extends to potential effects on synaptic plasticity. In fact in the DG of Ptch1+/– mice we detected deregulation of synaptic function-related genes (Grin1, Dlg4, Apbb1, Chrm2) that are concordant with the presence of proteins of the Shh pathway at synapsis (Petralia et al., 2011), which suggests a role for this morphogen as a synaptic function regulator.
Orphan nuclear receptor (TLX) is another central regulator of hippocampal neurogenesis, controlling the expression of a network of target genes to establish the undifferentiated and self-renewable state of NSCs (Shi et al., 2004). As transgenic TLX expression in mice led to enlarged brains and elongated hippocampal DG (Murai et al., 2014), a phenotype strongly resembling those of the Ptch1+/− mice, we evaluated its dependence from Shh pathway by evaluating TLX expression in Ptch1+/− mice. We detected increased TLX expression in 2 and 8 months old Ptch1+/− mice, suggestive of a dependence of TLX expression on Shh pathway activation. The identification of TLX upregulation (Antonelli et al., 2018) might suggest that the interplay between Shh and Notch signaling pathways converges on TLX to regulate the balance between proliferative and neurogenic division during hippocampal neurogenesis. TLX upregulation might concur to the morphological defects and deficit in adult neurogenesis in the DG of Ptch1+/− mice by altering the balance between stem cells maintenance and neuronal differentiation. This is supported by the findings that TLX is a direct target of Notch1/RBPJ in adult NSCs (Li et al., 2012) and it is positively regulated by Sox2 (Shimozaki et al., 2012).
Overall, we show that Shh-dependent morphological, cellular and molecular DG alterations, including deficit in newborn and mature neurons and deregulation of function-related synaptic genes are associated with altered behaviour in Ptch1+/− mice (Antonelli et al., 2018). In particular Ptch1+/− mice exhibit hypoactivity and decreased anxiety-related behaviors in the open field and elevated plus maze tests. Importantly, TLX is a known regulator of cognitive and anxiety-related behaviors and hyperactivity have been documented in TLX knockout mice, pointing to a possible molecular mechanism involving TLX upregulation in behavioral deficit in Ptch1+/− mice (O’Leary et al., 2016). In summary, modulation of the Shh pathway leads to morphological and functional defects in DG of adult Ptch1+/−, suggesting a central role for Shh in the regulation of hippocampal neurogenesis throughout mice adult life. Interactions with Notch signaling pathway result in the upregulation of TLX transcription factor, known to be critical for balancing NSCs proliferation and differentiation. Dysregulation of TLX might also concur to cause changes in mouse behaviour (Figure 2). Studies examining the causal relationship between hippocampal neurogenesis and cognitive disorders are fundamental to clarify the mechanisms leading to disease. Mechanistic understanding of cellular/molecular neurogenic process in health and disease is crucial for developing new treatments for a range of neurological conditions. Dysregulation of Shh signaling in brain leads to a wide range of neurological disorders including autism, depression, dementia, stroke, epilepsy, Parkinson’s and Huntington’s diseases, as well as brain tumors. A thorough understanding of the role of Shh pathway in the regulation of adult neurogenesis is need to establish whether modulation of the pathway may hold therapeutic benefits in neurological disorders.
Figure 2.
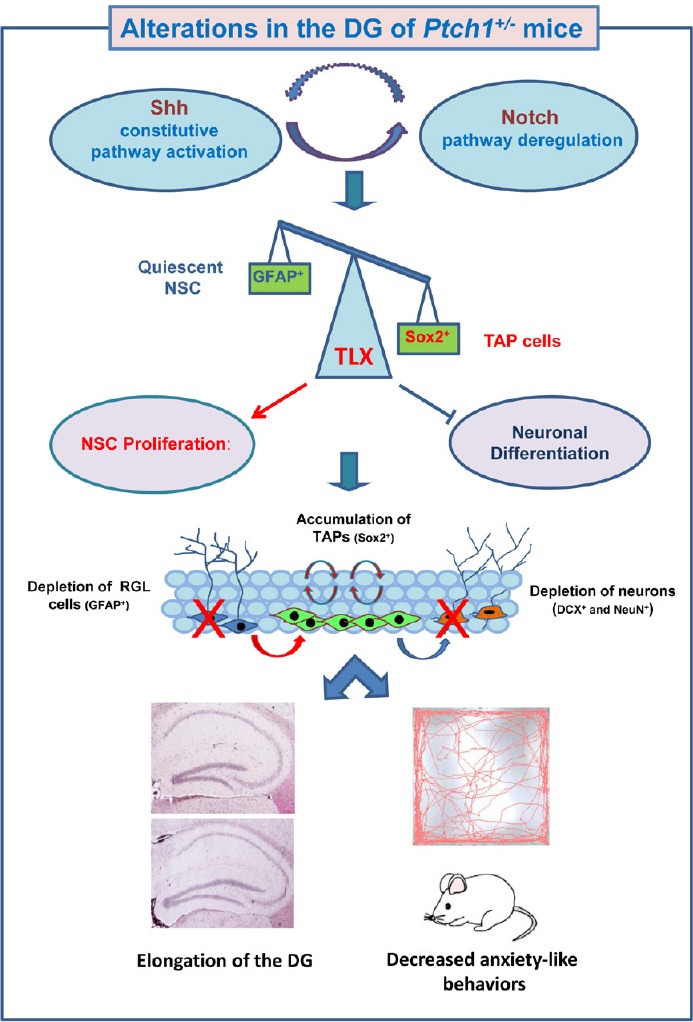
Sonic Hedgehog (Shh)-dependent morphological, cellular and molecular alterations in the dentate gyrus (DG) of Ptch1+/– mice.
Schematic model in which Shh pathway activation positively feeds into the activities of the Notch signaling pathway resulting in upregulation of TLX, a direct target of Notch1/recombining binding protein suppressor of hairless (RBPJ), positively regulated by Sex determining region Y (SRY) box 2 (Sox2) and overexpressed in the DG of Ptch1+/– mice. TLX is a critical transcription factors for balancing neural stem cells (NSCs) maintenance with neuronal differentiation and its upregulation is likely to promote neurogenic division and repress terminal differentiation, leading to tumor antigen presenting (TAP) cells accumulation and depletion of newborn neurons and astrocytes. The outcomes of this dysregulation are cellular and molecular defects in the hippocampus of Ptch1+/– mice associated with DG elongation, and alterations in mouse behaviour. Significantly up- and down-regulated genes are indicated in red and blue, respectively. Activation (arrow lines); inhibition (cut lines). Modified from Antonelli et al. (2018). GFAP: Glial fibrillary acidic protein; RGL: radial glia-like cells; DCX: doublecortin; Ptch1: Patched1.
This work was partially supported by grant 15234 from the Associazione Italiana per la Ricerca sul Cancro (AIRC).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Antonelli F, Casciati A, Tanori M, Tanno B, Linares-Vidal MV, Serra N, Bellés M, Pannicelli A, Saran A, Pazzaglia S. Alterations in morphology and adult neurogenesis in the dentate gyrus of Patched1 heterozygous mice. Front Mol Neurosci. 2018;11:168. doi: 10.3389/fnmol.2018.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn H, Wojnowski L, Zimmer AM, Hall J, Miller G, Zimmer A. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nat Med. 1998;4:619–622. doi: 10.1038/nm0598-619. [DOI] [PubMed] [Google Scholar]
- 3.Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. discussion 235-241, 302-306. [PubMed] [Google Scholar]
- 4.Kong JH, Yang L, Dessaud E, Chuang K, Moore DM, Rohatgi R, Briscoe J, Novitch BG. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev Cell. 2015;33:373–387. doi: 10.1016/j.devcel.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Hibbs MA, Gard AL, Shylo NA, Yun K. Genome-wide analysis of N1ICD/RBPJ targets in vivo reveals direct transcriptional regulation of Wnt, SHH, and hippo pathway effectors by Notch1. Stem Cells. 2012;30:741–752. doi: 10.1002/stem.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murai K, Qu Q, Sun G, Ye P, Li W, Asuelime G, Sun E, Tsai GE, Shi Y. Nuclear receptor TLX stimulates hippocampal neurogenesis and enhances learning and memory in a transgenic mouse model. Proc Natl Acad Sci U S A. 2014;111:9115–9120. doi: 10.1073/pnas.1406779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary JD, Kozareva DA, Hueston CM, O’Leary OF, Cryan JF, Nolan YM. The nuclear receptor Tlx regulates motor, cognitive and anxiety-related behaviours during adolescence and adulthood. Behav Brain Res. 2016;306:36–47. doi: 10.1016/j.bbr.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Petralia RS, Wang YX, Mattson MP, Yao PJ. Sonic hedgehog distribution within mature hippocampal neurons. Commun Integr Biol. 2011;4:775–777. doi: 10.4161/cib.17832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y, Chichung Lie D, Taupin P, Nakashima K, Ray J, Yu RT, Gage FH, Evans RM. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 11.Shimozaki K, Zhang CL, Suh H, Denli AM, Evans RM, Gage FH. SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem. 2012;287:5969–5978. doi: 10.1074/jbc.M111.290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33:1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]


