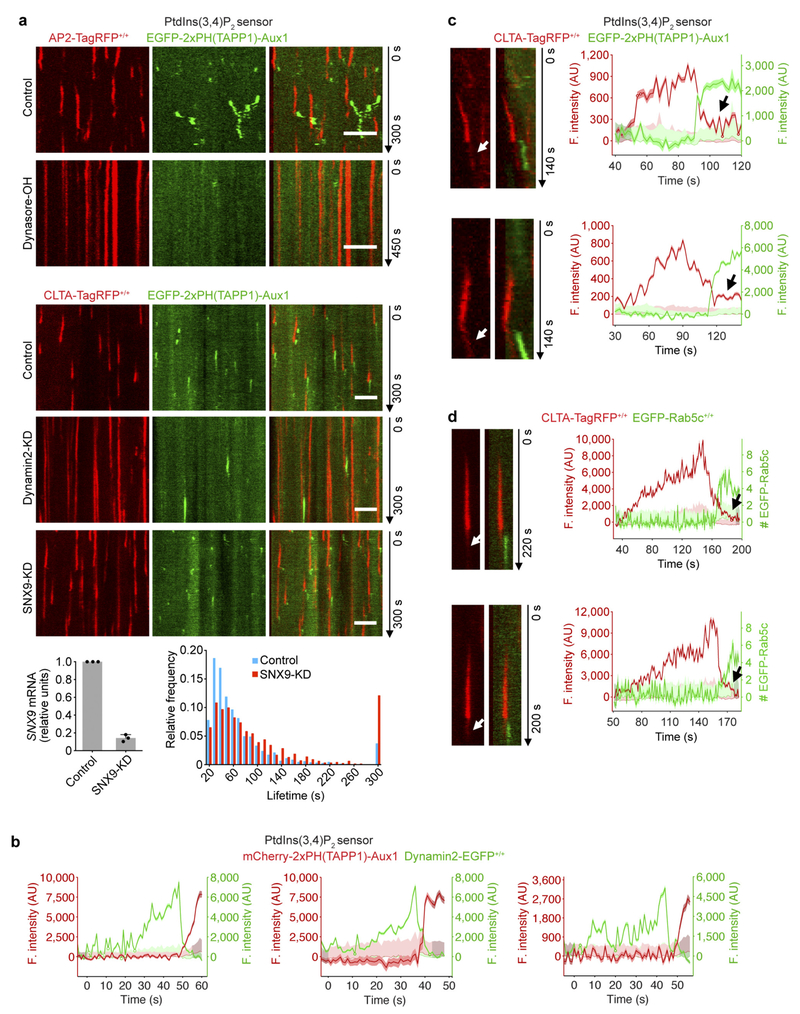
Extended Data Figure 5 |. Association of the Auxl-based PtdIns(3,4)P2 sensor together with a few copies of clathrin with uncoated vesicles.
a, Blocking by the dynamin inhibitor dynasore-OH of accumulation of the PtdIns(3,4)P2 sensor on clathrin-coated structures in AP2-TagRFP+/+ cells transiently expressing the PtdIns(3,4)P2 sensor EGFP-2 × PH(TAPP1)-Aux1 (top). The cells were incubated for 10 min without or with 30 μM dynasore-OH and then imaged by spinning-disk confocal microscopy at the bottom surface. The representative kymograph highlights the expected stalling of coated pits induced by brief exposure to dynasore-OH together with their failure to recruit the PtdIns(3,4)P2 sensor. CLTA-TagRFP+/+ cells stably expressing the PtdIns(3,4)P2 sensor EGFP-2×PH(TAPP1)-Aux1 were treated with siRNA targeting dynamin2 or SNX9, or with a control sequence, and imaged by TIRF microscopy (bottom). The kymograph from a time series of a cell depleted of dynamin2 shows that the stalled (that is, persistent) coated pits lack the PtdIns(3,4)P2 sensor. The bottom panels show the efficiency of depletion of SNX9 mRNA measured by real-time quantitative PCR (n = 3 independent experiments, mean ± s.d.) and the distributions of lifetimes for clathrin-coated structures in control (1,513 traces from 8 cells) and SNX9-KD cells (1,038 traces from 11 cells). b, Representative fluorescence traces obtained from gene-edited dynamin2-EGFP+/+ SUM159 cells transiently expressing the Aux1-based PtdIns(3,4)P2 sensor mCherry-2× PH(TAPP1)-Aux1 imaged by TIRF microscopy. Recruitment of the PtdIns(3,4)P2 sensor follows loss of the dynamin signal corresponding to the membrane scission associated with coated pit budding and formation of a coated vesicle. c, Temporal relationship between recruitment of the Auxl-based PtdIns(3,4)P2 sensor and the onset of uncoating of endocytic clathrin-coated vesicles. CLTA-TagRFP+/+ cells transiently expressing the PtdIns(3,4)P2 sensor EGFP-2×PH(TAPP1)-Aux1 were imaged by spinning-disk confocal microscopy towards the leading edge of their bottom surface. Tracking endocytic events near the leading edge of the cell where the uncoated vesicles show limited vertical movement enabled us to capture examples of endocytic events still containing a small number of clathrin triskelions associated with uncoated vesicles (arrows). The representative kymographs (left) and corresponding fluorescence intensity traces (right) from naked vesicles show association of the PtdIns(3,4)P2 sensor together with a few copies of clathrin (arrow) after culmination of the uncoating process. d, Double gene-edited CLTA-TagRFP+/+ and EGFP-Rab5c+/+ SUM159 cells were imaged by TIRF microscopy at the leading edge of their bottom surface. Representative kymographs (left) and corresponding fluorescence intensity traces (right) highlight that a small number of clathrin triskelions remained associated with the uncoated vesicles (arrows). Temporal relationship showing that onset of Rab5c recruitment occurred after the uncoating of endocytic clathrin-coated vesicles. The EGFP channel in all the kymographs with EGFP and TagRFP overlaid was shifted laterally by six pixels. Data are representative of at least two independent experiments. Scale bars, 5 μm.