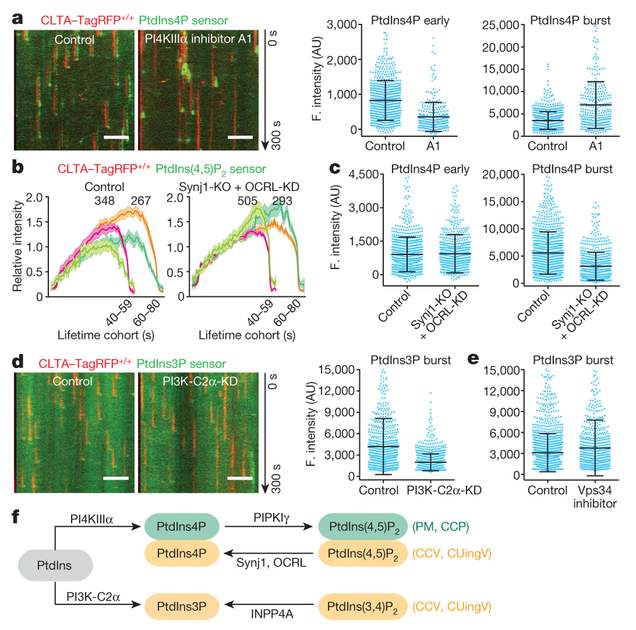
Figure 3 |. Interference of phosphoinositol kinases and phosphatases on phosphoinositide content of endocytic clathrin-coated structures.
a, Inhibition of PI4KIIIα lowered accumulation of PtdIns4P sensor during coated pit assembly. CLTA-TagRFP+/+ cells stably expressing the PtdIns4P sensor EGFP-P4M(DrrA)-Aux1 were treated with dimethyl sulfoxide (DMSO; control) or the PI4KIIIα inhibitor A1. Representative kymographs and sensor intensities from 738 and 303 traces from 5 control and 14 treated cells, respectively. A1 significantly decreased early PtdIns4P content (Cohen’s d = 0.89) and increased burst amplitude (Cohen’s d = 1.06). b, Eliminating Synj1 and depleting OCRL by 80% increased recruitment of the PtdIns(4,5)P2 sensor to clathrin-coated structures. The PtdIns(4,5)P2 sensor EGFP-PH(PLCδ1)-Aux1 was transiently expressed in control or Synj1-knockout (KO) CLTA-TagRFP+/+ cells treated with control or OCRL siRNA (OCRL-KD). Plots show averaged fluorescence (F.) intensity traces of clathrin and the PtdIns(4,5)P2 sensor associated with endocytic coated pits, including the numbers of analysed traces from 10 control and 11 Synj1-KO + OCRL-KD cells. c, Synj1-KO + OCRL-KD diminished PtdIns4P sensor burst during uncoating (Cohen’s d = 0.72) but had no effect on early PtdIns4P content of coated pits (Cohen’s d = 0.04); 895 and 704 traces from 9 control and 11 Synj1-KO + OCRL-KD cells, respectively. d, PI3K-C2α knockdown in CLTA-TagRFP+/+ cells stably expressing PtdIns3P sensor EGFP-2×FYVE(Hrs)-Aux1 significantly decreased the late burst of sensor (Cohen’s d = 0.81); 769 and 1,004 traces from 11 control and 13 PI3K-C2α-KD cells, respectively. e, Inhibition of Vps34 by PIK-III in CLTA-TagRFP+/+ cells expressing the PtdIns3P sensor EGFP-2×FYVE(Hrs)-Aux1 had a minor effect on sensor burst (Cohen’s d = 0.21); 1,695 and 742 traces from 7 control and 7 treated cells, respectively. f, Phosphoinositide kinases and phosphatases responsible for phosphoinositide interconversion in clathrin-mediated endocytic traffic. CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; CUingV, clathrin-coated vesicle undergoing uncoating; PM, plasma membrane. Data from TIRF microscopy (300 s, 1-s intervals); EGFP signal in the kymographs laterally shifted six pixels. Scale bars, 5 μm. See Methods for statistics.