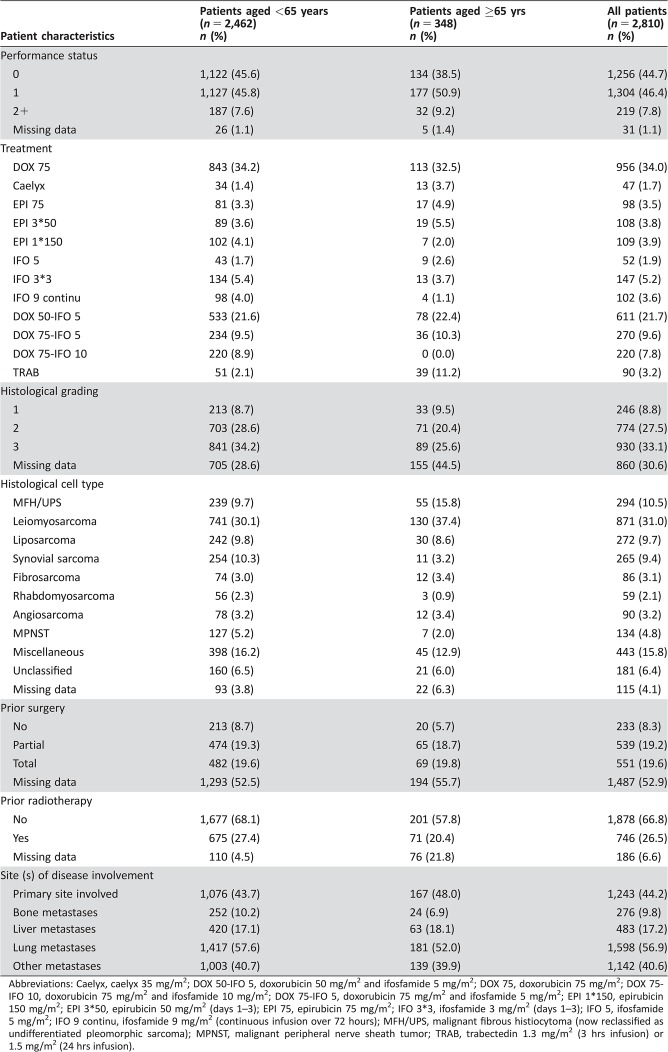
Table 1. Patient characteristics.
Abbreviations: Caelyx, caelyx 35 mg/m2; DOX 50‐IFO 5, doxorubicin 50 mg/m2 and ifosfamide 5 mg/m2; DOX 75, doxorubicin 75 mg/m2; DOX 75‐IFO 10, doxorubicin 75 mg/m2 and ifosfamide 10 mg/m2; DOX 75‐IFO 5, doxorubicin 75 mg/m2 and ifosfamide 5 mg/m2; EPI 1*150, epirubicin 150 mg/m2; EPI 3*50, epirubicin 50 mg/m2 (days 1–3); EPI 75, epirubicin 75 mg/m2; IFO 3*3, ifosfamide 3 mg/m2 (days 1–3); IFO 5, ifosfamide 5 mg/m2; IFO 9 continu, ifosfamide 9 mg/m2 (continuous infusion over 72 hours); MFH/UPS, malignant fibrous histiocytoma (now reclassified as undifferentiated pleomorphic sarcoma); MPNST, malignant peripheral nerve sheath tumor; TRAB, trabectedin 1.3 mg/m2 (3 hrs infusion) or 1.5 mg/m2 (24 hrs infusion).