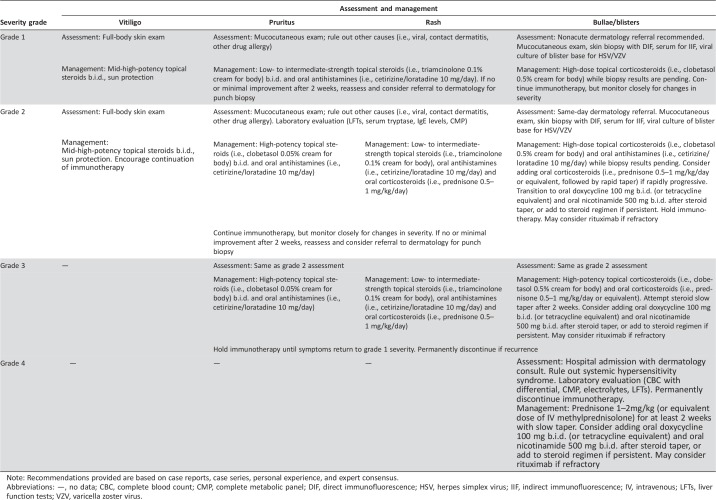
Table 2. Columbia University Cutaneous Oncology Center recommendations for management of Common Terminology Criteria for Adverse Events‐based immune‐related adverse events associated with immune checkpoint inhibitors.
Note: Recommendations provided are based on case reports, case series, personal experience, and expert consensus.
Abbreviations: —, no data; CBC, complete blood count; CMP, complete metabolic panel; DIF, direct immunofluorescence; HSV, herpes simplex virus; IIF, indirect immunofluorescence; IV, intravenous; LFTs, liver function tests; VZV, varicella zoster virus.