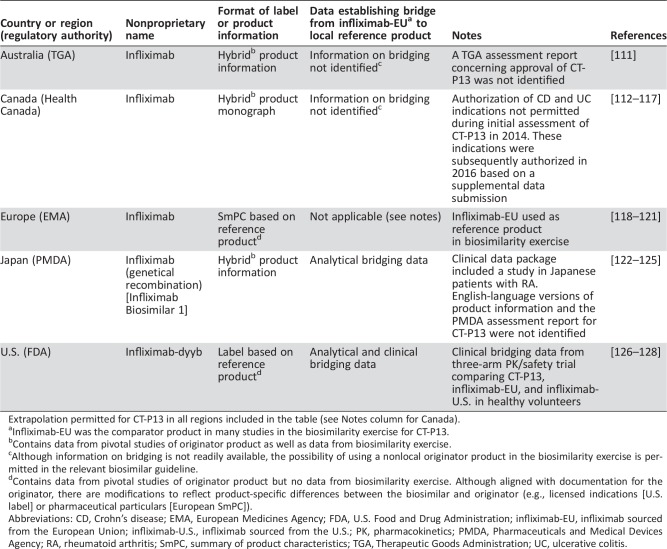
Table 1. Attributes of biosimilar infliximab CT‐P13 in different regulatory regions.
Extrapolation permitted for CT‐P13 in all regions included in the table (see Notes column for Canada).
Infliximab‐EU was the comparator product in many studies in the biosimilarity exercise for CT‐P13.
Contains data from pivotal studies of originator product as well as data from biosimilarity exercise.
Although information on bridging is not readily available, the possibility of using a nonlocal originator product in the biosimilarity exercise is permitted in the relevant biosimilar guideline.
Contains data from pivotal studies of originator product but no data from biosimilarity exercise. Although aligned with documentation for the originator, there are modifications to reflect product‐specific differences between the biosimilar and originator (e.g., licensed indications [U.S. label] or pharmaceutical particulars [European SmPC]).
Abbreviations: CD, Crohn's disease; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; infliximab‐EU, infliximab sourced from the European Union; infliximab‐U.S., infliximab sourced from the U.S.; PK, pharmacokinetics; PMDA, Pharmaceuticals and Medical Devices Agency; RA, rheumatoid arthritis; SmPC, summary of product characteristics; TGA, Therapeutic Goods Administration; UC, ulcerative colitis.