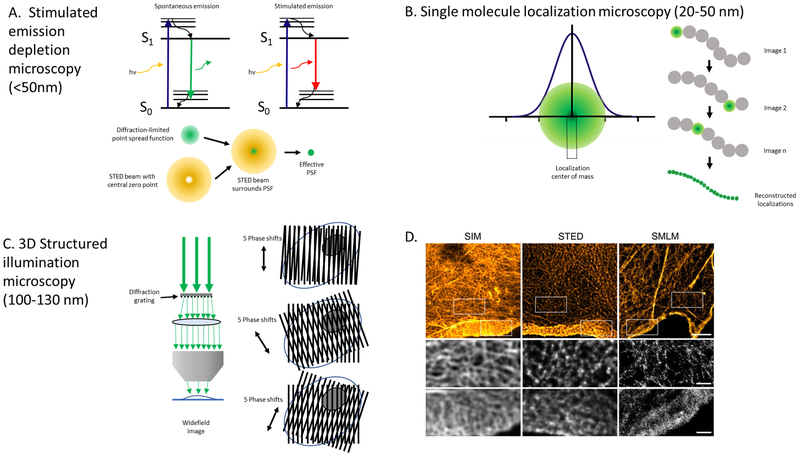
Figure 2. Super-resolution microscopy techniques.
The fluorescence and optical principles for three super-resolution microscopy techniques are shown with demonstrated lateral resolution noted in parentheses. (A) Stimulated emission depletion microscopy (STED): Effectively reduces the volume of the point spread function (PSF) of an objective lens, which is the three-dimensional diffraction volume of emitted light transmitted by an objective lens from an infinitesimally small point source of light in the specimen. A red-shifted laser with hollow beam (depletion laser) quenches fluorophores on the periphery of the PSF, leaving a small central subdiffraction volume of excited fluorophores that emit photons. Adapted from Blom and Widegren, 2017. (B) Single molecule localization microscopy (SMLM): Encompasses several related techniques that obtain many thousands of images of a given sample in which only a very small number of fluorophores are fluorescing at a given time. Precise localization of the geometric centers of individual fluorophores within the thousands of collected images can then be used to reconstruct a super-resolution image. (C) Structured illumination microscopy (SIM): A moveable diffraction grating is placed in the illumination aperture of the excitation beam path of a laser-illuminated wide-field microscopy set-up to generate a sinusoidal pattern of light with resulting Moiré fringes. The high frequency illumination stripes and high frequency object organization create even higher spatial frequencies below the diffraction limit. Multiple raw images must be collected at different diffraction orientations to reconstruct the final super-resolved image. (D) Actin cytoskeleton of COS-7 cells labeled with phalloidin-AlexaFluor 488 using SIM and STED and phalloidin-AlexaFluor647 using SMLM. Boxed areas note higher resolution areas of the lamella and lamellipodium in each optical section. Reprinted with permission from Wegel et al., 2016.