Table 6.
Substrate scopes for L-proline-catalyzed α-aminoxylation of aldehydes with nitrosobenzene.
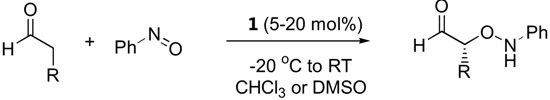 |
| R | conditions | yield (%)a | ee (%)a | ref. |
|---|---|---|---|---|
| Me, Et, nPr, iPr, Ph, Bn | 30 mol% 1, MeCN, -20 ºC, 24 h | 62–>99 | 95–99 | [62] |
| Me, nBu, iPr, allyl, Bn, Ph, TIPSO(CH2)3, N-methylindol-3-ylmethyl | 5 mol% 1, CHCl3, 4 ºC, 4 h | 60–88 | 97–99 | [63] |
| Me, nPr, iPr, nBu, allyl, Bn, BnOCH2, BocNH(CH2)4 | 20 mol% 1, DMSO, RT, 10–20 min | 54–86 | 94–99 | [64] |
a Yield and ee were determined after NaBH4 reduction to the corresponding diols.
