Table 8.
Comparison of catalysts in aminoxylation/nitrosoaldol reactions of aldehydes.
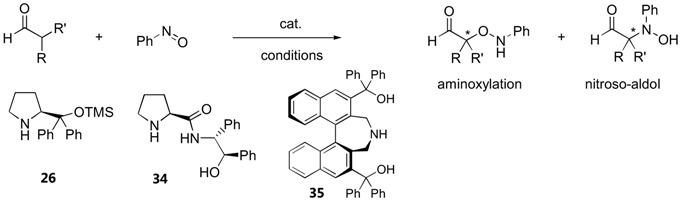 |
| Cat. | Substratea | Conditions | Yield (%) | ee (%)b | Ref. |
|---|---|---|---|---|---|
| 1 | 2-methyl-3-propanal | 20 mol% cat., DMF, | 32 | 67 | [114] |
| 25 ºC, 24 h | (N:O = 1.5:1) | ||||
| 2 | α-branched aldehydes (10) | 20 mol% cat., DMF, | 55–96 | 5–90 | [114] |
| 0–25 ºC , 3–24 h | (N:O = 0.6:1–20:1) | ||||
| 34 | α-branched aldehydes (5) | 10 mol% cat., toluene, | 53–74 | 46–59 | [115] |
| -40 ºC, 2–3 d | (N:O = N/A) | ||||
| 26 | α-unbranched aldehydes (8) | 20 mol% cat., CH2Cl2, | 40–75 | 91–99 | [116] |
| 0 ºC | (N:O >99:1) | ||||
| 35 | α-unbranched aldehydes (6) | 10 mol% cat., THF, | 70–90 | 96–99 | [117] |
| 0 ºC, 1 h | (N:O >99:1) |
a Number of examples shown in parentheses bee of the N-nitrosoaldol product.
