Figure 5: Oral gavage of SRM1649b PM, in the absence of AHR ligands in the diet, has no effect on severity of EAE at high or low doses in vivo.
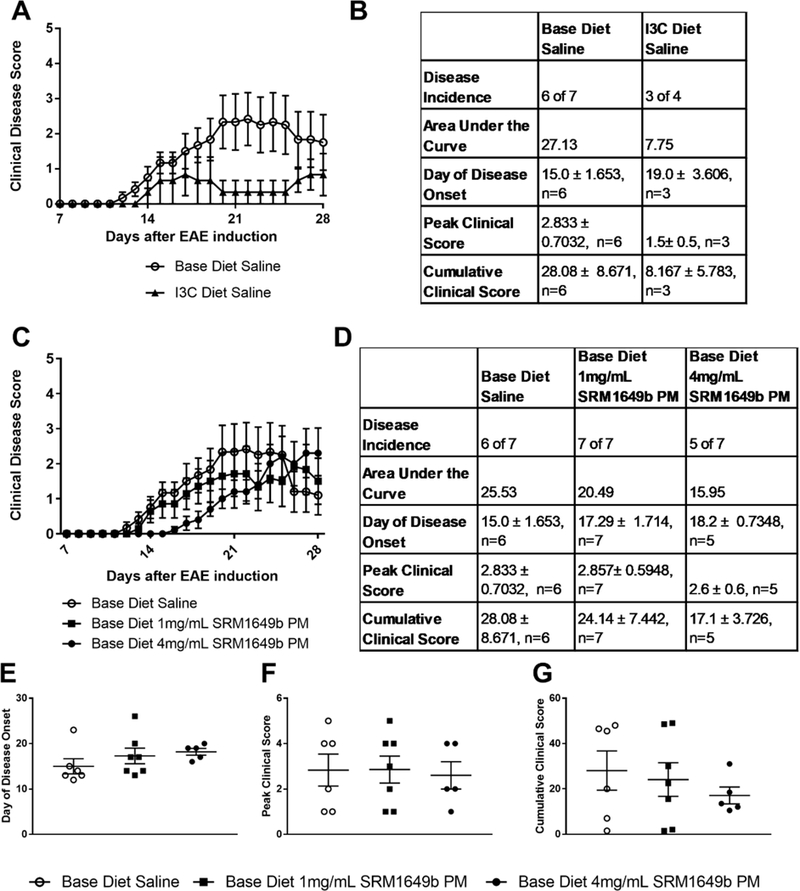
Age matched female WT (C57BL/6J) mice were started on base diet (no AHR ligands) or I3C (enriched AHR ligands) at day −38. Mice received 4.0mg/mL PM (800μg per dose) or 1.0mg/mL PM (200μg per dose) of SRM1649b PM or Saline control by oral gavage 8 times starting at day −12 every 3 days until day 9 after induction. Mice were weighed and scored starting on day 7 until day 28. (A, B) Mice were placed on base or I3C diet and administered saline by gavage. I3C diet lessened severity of EAE. (C, D) Mice on base diet were administered 4.0 or 1.0mg/mL SRM1649b PM or Saline control by oral gavage. High or low dose SRM1649b PM did not worsen severity of EAE. (E) There was no difference in day of disease onset at high or low doses of SRM1649b PM after gavage (F) There was no difference in peak clinical score at either dose of SRM1649b PM. (G) There was no difference in cumulative clinical score at either dose of SRM1649b PM. Results are mean ± SEM of (Base diet Saline n=6), (I3C diet n=3), (Base diet SRM1649b PM 4mg/mL PM, n=5), and (Base diet SRM1649b PM 1mg/mL PM, n=7). Significant differences among groups (p<0.05) are indicated by an asterisk. Abbreviations: SRM, standard reference materials; PM, particulate matter; I3C, indole-3-carbinol; AHR, aryl hydrocarbon receptor; EAE, experimental autoimmune encephalomyelitis; SEM, standard error of the mean.
