Figure 7.
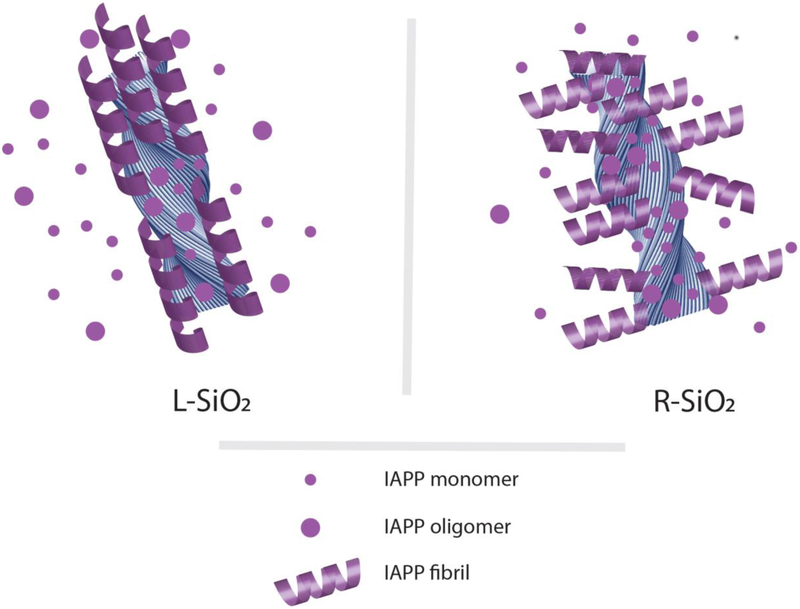
Adsorption of IAPP monomers and oligomers accelerated their nucleation and aggregation on silica nanoribbon surfaces. Such interactions decreased the net concentrations of free IAPP monomers and oligomers in solution to mitigate their associated toxicity. Due to the steric constraints imposed by the chiral silica nanoribbons, left-handed IAPP amyloid fibrils rendered by surface-adsorbed monomers and oligomers elongated away from or perpendicular to the R-SiO2 backbone. In contrast, left-handed IAPP fibrils extended nearly parallel to the L-SiO2 surfaces, thereby shielding the nanostructures from being further accessed by the peptide. Accordingly, the R-SiO2 possessed much more binding sites per surface area than the L-SiO2 for IAPP adsorption and aggregation, as also evidenced by TEM and DMD simulations, and hence were far more effective in preventing IAPP induced toxicity.