Figure 2.
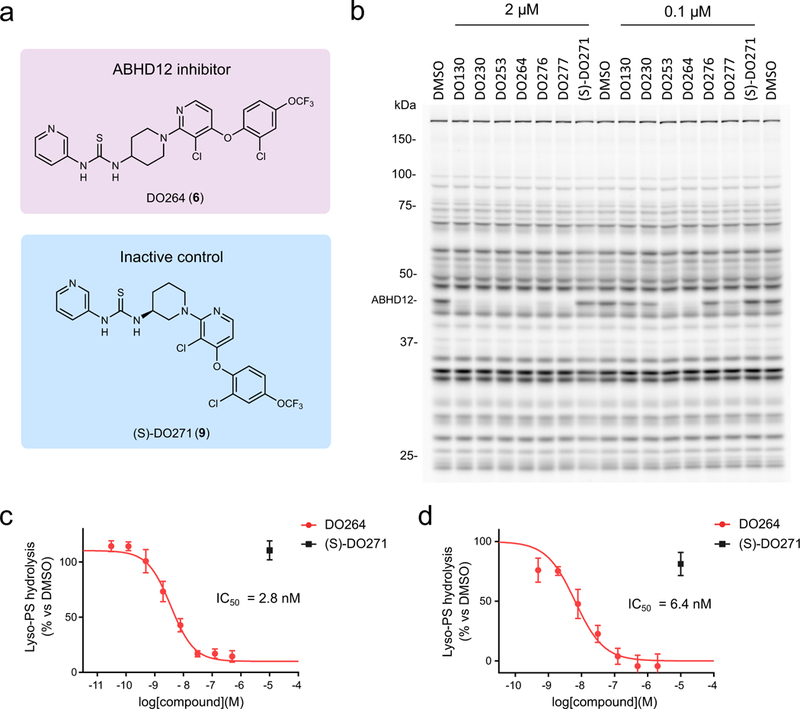
Discovery and optimization of (thio)urea inhibitors of ABHD12. (a) Chemical structures of an optimized ABHD12 inhibitor DO264 and an inactive control compound (S)-DO271. (b) In vitro potency and selectivity of (thio)urea ABHD12 inhibitors and the control compound (S)-DO271 (0.1 or 2 μM, 30 min pre-incubation 37 oC) measured in mouse brain membrane proteome (1 mg/mL) by gel-based competitive ABPP using the FP-Rh probe (1 μM, 45 min, 37 °C). The result is a representative of two independent experiments. (c, d) Concentration-dependent inhibition of lyso-PS hydrolysis activity of mouse brain (c) or THP-1 (d) membrane proteome (0.4 mg/mL) by DO264 as determined using 17:1 lyso-PS as a substrate. Data were normalized to ABHD12(−/−) brain and JJH350 (10 μM)-treated THP1 membrane proteomes, respectively, to designate the ABHD12-dependent component of lyso-PS hydrolysis in these proteomes (see Supplementary Fig. 9) and represent average values ± SD. n = 4 and 3 independent experiments for c and d, respectively.
