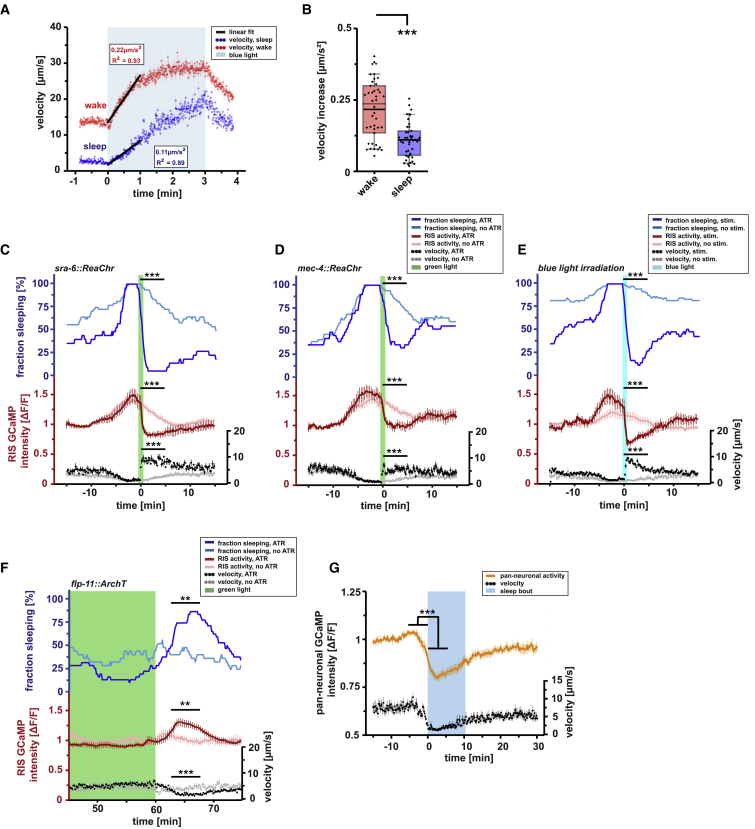
Figure 3.
Behavioral Quiescence during L1 Arrest Presents a Sleep State
(A and B) To test for responsiveness to stimulation during quiescence in arrested L1 larvae (A), a blue light stimulus was given and locomotion velocity was measured. (B) Linear regression of locomotion speeds during the first minute of blue light irradiation was used to measure locomotion acceleration. Waking worms (red dotted line, red box) accelerated with 0.22 ± 0.02 μm/s2, whereas sleeping worms (blue dotted line, blue box) accelerated only with 0.11 ± 0.01 μm/s2 (n = 42 worms; ∗∗∗p < 0.001; paired Wilcoxon rank test).
(C–E) To test for sleep reversibility, nociceptive ASH neurons and mec-4 expressing mechano-sensory neurons were stimulated optogenetically with ReaChr and green light in sleeping arrested L1 larvae. In addition, arrested L1 larvae were stimulated with noxious blue light. RIS activity is shown in red (control without all trans-Retinal [ATR] in light red), speed in black (control without ATR in gray), and the fraction of sleeping animals in blue (control without ATR in light blue).
(C) Sleeping worms responded immediately to ASH activation and the sleep bout ceased. The fraction of sleeping animals decreased by 91.6% ± 1.7% (control worms without ATR by 12.3% ± 2.9%; ∗∗∗p < 0.001; two-sample t test). RIS activity (ΔF/F) dropped by 35.5% ± 4.2% (control worms without ATR showed only a drop of ΔF/F of 11.9% ± 0.3%; ∗∗∗p < 0.001 two-sample t test). Locomotion velocity increased by 313.7% ± 26.0% (control worms without ATR by 65.7% ± 14.0%; ∗∗∗p < 0.001; two-sample t test). n(ATR) = 23 worms; n(without ATR) = 47 worms.
(D) Sleeping worms immediately responded to mec-4-expressing mechano-sensory neuron activation, and the sleep bout ceased. The fraction of sleeping animals decreased by 52.45% ± 6.0% (control worms without ATR by 10.0% ± 2.6%; ∗∗∗p < 0.001; two-sample t test). RIS activity (ΔF/F) dropped by 32.8% ± 3.2% (control worms without ATR showed only a drop of ΔF/F of 7.0% ± 2.1%; ∗∗∗p < 0.001; two-sample t test). Locomotion velocity increased by 351.7% ± 44.0% (control worms without ATR by 14.6% ± 4.0%; ∗∗∗p < 0.001; two-sample t test). n(ATR) = 29 worms; n(without ATR) = 50 worms.
(E) Sleeping worms immediately responded to noxious blue light illumination, leading to sleep bout termination. The fraction of sleeping animals decreased by 75.5% ± 3.5% (control worms without blue light stimulation by 4.1% ± 0.6%; ∗∗∗p < 0.001; two-sample t test). RIS activity (ΔF/F) dropped by 43.2% ± 7.9% (control worms without blue light stimulation showed only a drop of ΔF/F of 4.3% ± 7.7%; ∗∗∗p < 0.001; two-sample t test). Locomotion velocity increased by 367.9% ± 5.8% (control worms without ATR by 80.3% ± 28.2%; ∗∗∗p < 0.001; two-sample t test). n(stimulation) = 35 worms; n(without stimulation) = 37 worms.
(F) To test for homeostasis, RIS was inhibited for 1 hr with ArchT and green light in the arrested L1 larvae. RIS activity is shown in red (control without ATR in light red), speed in black (control without ATR in gray), and the fraction of sleeping animals in blue (control without ATR in light blue). After the end of green light exposure, the fraction of sleeping worms increased by 265.6% ± 13.1% (control worms without ATR decreased by 19.3% ± 6.7%; ∗∗p = 0.007; two-sample t test). RIS activity (ΔF/F) increased by 24.3% ± 2.2% (in control worms without ATR, it increased by 5.6% ± 1.0%; ∗∗p = 0.005; two-sample t test). Locomotion velocity decreased by 63.3% ± 16.0% (control worms without ATR increased by 5.5% ± 4.0%; ∗∗∗p < 0.001; two-sample t test). n(ATR) = 32 worms; n(without ATR) = 25 worms.
(G) To test for overall neuronal activity during a sleep bout, calcium activity in the head neurons was measured using pan-neuronal GCaMP6s. Head neuron activity is shown in orange and locomotion speed in black; blue shading shows sleep bouts as defined by a locomotion cessation threshold. At the onset of a sleep bout, head neuron activity decreased and locomotion ceased. ΔF/F was decreased to 82.3% ± 0.2% (n = 21 worms; ∗∗∗p < 0.001; paired Wilcoxon rank test).
Error bars in (C)–(G) indicate the SEM. See also Figure S3.