Table 2.
Inhibitory activities against Topo I and cytotoxicities of derivatives of luotonin A on ring A and B.
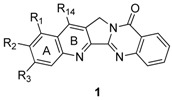 |
| Compd | R1 | R2 | R3 | R14 | Inhibitory activity against Topo I [% (rel. activity)] at 100 μM | IC50 (μM) | ||
|---|---|---|---|---|---|---|---|---|
| HCT-116 | HL-60 | H460 | ||||||
| 1aa | H | H | H | H | 33.7 (0.40) [56] | 51.11 [56] | >100 [56] | 7.7 [58] a) |
| 1d | H | H | F | H | 81.1 (0.91) [56] | >100 [56] | 56.65 [56] | - |
| 1e | H | OH | H | H | 54.1 (0.65) [56] | 56.6 [56] | 66.29 [56] | - |
| 1f | H | OMe | OMe | H | - | - | - | 5.47 [58] |
| 1g | H | -OCH2CH2O- | H | 19.2 (0.23) [56] | 19.36 [56] | 21.78 [56] | 81 [58] | |
| 1h | H | R c) | H | H | - | - | 2 [40] | - |
| 1i | H | R d) | H | H | - | - | 2 [40] | - |
| 1j | OH | R e) | H | H | - | - | 10 [40] | - |
| 1k | H | H | H | Et | slightly more active b)[41] | - | - | - |
| 1l | H | OH | H | Et | similar to luotonin A [41] | - | - | - |
| 1m | H | H | H | CF3 | inhibition at 40 μM [59] | - | - | - |
| Doxo f) | - | 2.31 [56] | 4.78 [56] | - | ||||
| CPT | 83.9 (1) [56] | 2.17 [56] | 5.51 [56] | - | ||||
a) Values obtained from 72 h culture, b) no detailed data were available [41], c) R = OCH2CH2NEt2, d) OCH2CH2NMe2, e) CH2NMe2, f) doxorubicin.
