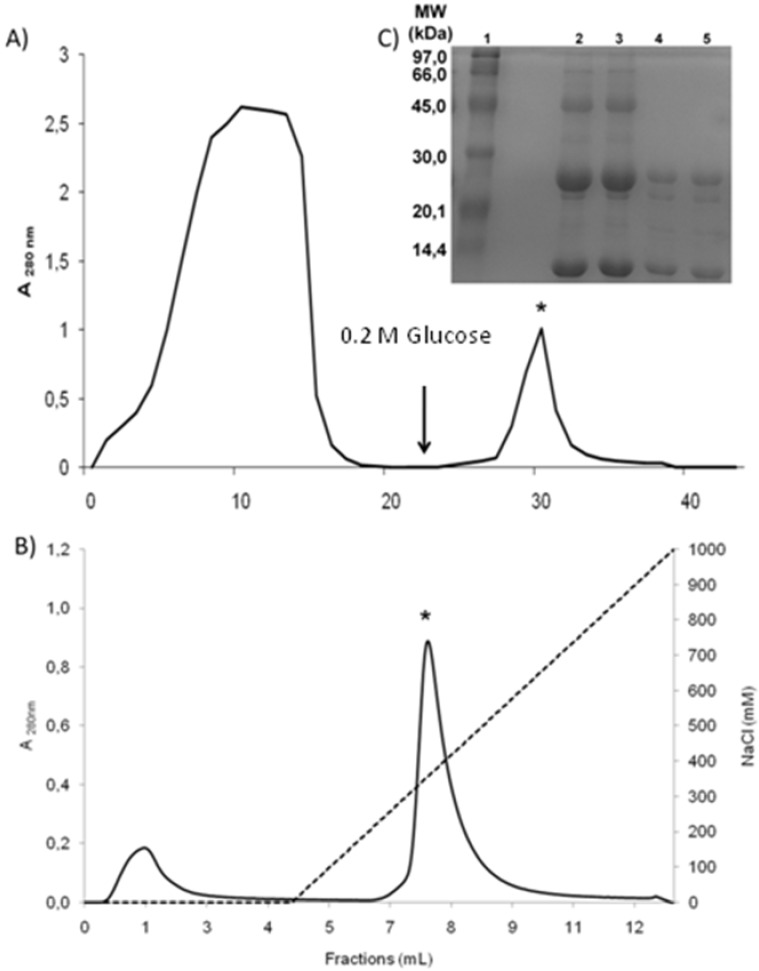
Figure 1.
Purification of lectin from the seeds of Dioclea wilsonii (DwL). (a) Partial purification of DwL by affinity chromatography on a Sephadex G-50 column. The column was equilibrated and eluted with 0.1 M Tris-HCl buffer (pH 7.4) containing 0.15 M NaCl to remove the unbound proteins. Lectin (*Peak II) was recovered with 0.2 M Glucose or with 0.1 M Glycine buffer (pH 2.6) containing 0.15 M NaCl. (b) Purification of DwL by cation exchange chromatography on a SP Sepharose™ XL column using peak II from the affinity chromatography step. The column was equilibrated and eluted by a saline gradient (0–1 M NaCl) to remove contaminants (Peak I). Lectin (*Peak II) was recovered with Acetate buffer (pH 4.5). (c) SDS-polyacrylamide gel electrophoresis (15%) of DwL in the presence of β-mercaptoethanol. Lane 1: molecular mass markers [phophorylase b (97 kDa), albumin (66 kDa), ovalbumin (45 kDa), carbonic anhydrase (30 kDa), trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.4 kDa)]. Lanes 2 and 3: Peak II of the Sephadex G-50 column. Lanes 4 and 5: Peak II of the SP Sepharose™ XL column.