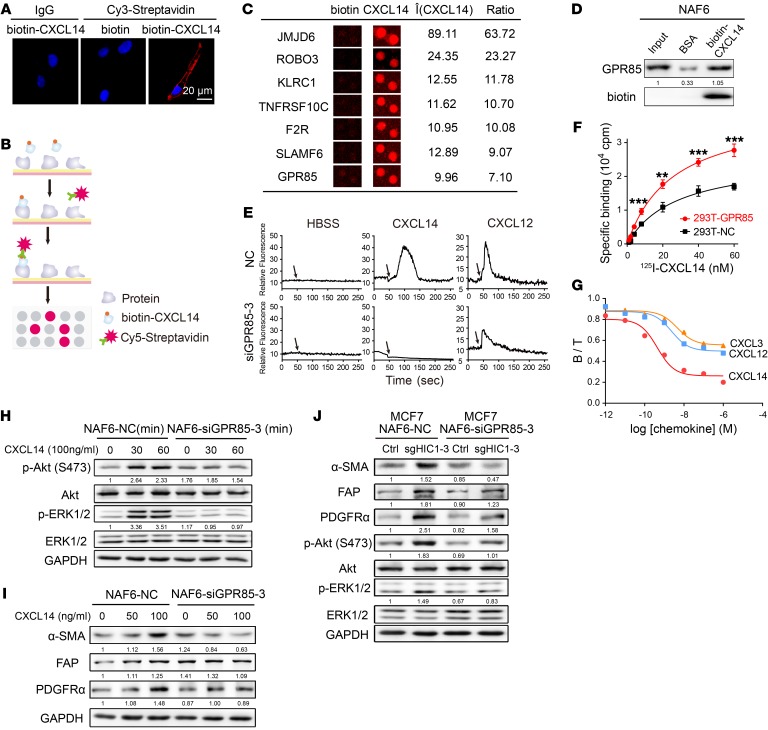
Figure 5. GPR85 is a functional receptor for CXCL14 activity.
(A) Confocal microscopy of NAF6 cells treated with 100 ng/ml biotin or biotin-CXCL14 at 4°C and stained with an antibody against Cy3-streptavidin. An isotype-matched IgG was used as a control. Cell nuclei were counterstained with DAPI. (B) Schematic of the procedure used to detect biotin-CXCL14–binding proteins using HuProt human proteome microarrays containing 18,583 affinity-purified N-terminally GST-tagged proteins. (C) Representative CXCL14-binding membrane proteins in the proteome microarrays. (D) Western blot validation using a streptavidin-agarose pull-down assay of proteome microarray determination that CXCL14 binds directly to GPR85. (E) Mobilization of [Ca2+]i in NAF6 cells that were transfected with control siRNA (NC) or GPR85-3 siRNA and then treated with 100 ng/ml HBSS, rhCXCL14, or rhCXCL12. The black arrows denote the times at which stimulation was initiated. (F) 125I-CXCL14 binding properties between 293T-NC and 293T-GPR85 cells. Data are shown as mean ± SD. n = 4 repetitions. **P < 0.01; ***P < 0.001, 2-tailed Student’s t test. (G) Binding assay with 10 nM 125I-CXCL14 in the presence or absence of increasing concentrations of unlabeled rhCXCL14, rhCXCL12, and rhCXCL3 for 293T cells that were transfected with GPR85. B, specific binding; T, total binding. (H) Knockdown of GPR85 expression by GPR85-3 siRNA in NAF6 cells in the presence or absence of 100 ng/ml rhCXCL14 for the indicated times (0, 30, and 60 minutes). Cell lysates were analyzed by Western blot with antibodies against p-Akt (Ser 473), Akt, p-ERK1/2, ERK1/2, and GAPDH. (I) Knockdown of GPR85 expression by GPR85-3 siRNA in NAF6 cells treated with rhCXCL14 at various concentrations (0–100 ng/ml) for 4 days. Cell lysates were analyzed by Western blot with antibodies against α-SMA, FAP, PDGFRα, and GAPDH. (J) NAF6 cells were transfected with control siRNA (NC) or GPR85-3 siRNA and then cocultured with MCF7Ctrl or MCF7sgHIC1 cells, respectively, for 4 days. NAF6 cell lysates were analyzed by Western blot with antibodies against α-SMA, FAP, PDGFRα, p-Akt (Ser 473), Akt, p-ERK1/2, ERK1/2, and GAPDH.