Table 1.
Diastereoselective addition of phenylacetylene in the presence of ligands.
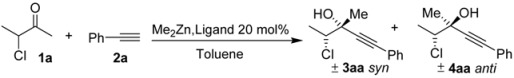 | |||
|---|---|---|---|
| Entry a | ligand | syn% | anti% |
| 1 b | -- | 48 | 52 |
| 2 | 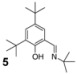 |
23 | 77 |
| 3 | 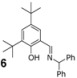 |
29 | 71 |
| 4 | 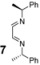 |
23 | 77 |
| 5 |  |
22 | 78 |
| 6 | 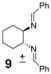 |
29 | 71 |
| 7 c | -- | 6 | 94 |
a All the reactions were performed at rt under nitrogen atmosphere Me2Zn (3 equiv.) and phenylacetylene (3 equiv.) were added to toluene in a flask under strictly anhydrous conditions, and stirred 10 min, then the ligand (20 mol%) was added and the reaction mixture and stirred for 5–10 min. Finally, the chloroketone (1 equiv.) was added. The reaction was stirred under completion (monitored by TLC, 16–24 h) and quenched with water. The dr was determined on the crude reaction mixture by 1H-NMR. b The reaction was performed in the presence of Me2Zn without ligands. c The reaction was performed with lithium phenylacetylide, prepared by the addition of 1 equiv. of n-BuLi to 1.1 equiv. of phenylacetylene at 0 °C.
