Table 3.
Stereoselective addition of phenylacetylene to a series of α-chloroketones promoted by (R,R)-Salen ligand.
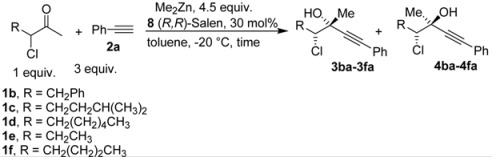 | ||||||
|---|---|---|---|---|---|---|
| Entry a | Ketone | dr b | T (h) | ee anti c | ee syn c | Yield d ( syn + anti) |
| 1 | 1a | 82:18 | 60 | 90 | 82 | 26 |
| 2 | 1b | 73:27 | 120 | 85 | 65 | 35 |
| 3 | 1c | 77:23 | 60 | 81 | 58 | 25 |
| 4 | 1d | 78:22 | 60 | 82 | 55 | 18 |
| 5 | 1e | 75:25 | 140 | 62 | 42 | 40 |
| 6 | 1f | 80:20 | 60 | 83 | 57 | 17 |
a All the reactions were performed at −20 °C under nitrogen and stopped after the time indicated. b The diastereoisomeric ratios were evaluated on the crude reaction mixture by 1H-NMR. c The enantiomeric excess was evaluated by chiral HPLC analysis (see experimental part for the conditions) d Isolated yield (syn + anti) after chromatographic purification.
