Table 1.
The basic set of η6-arene/N-sulfonyldiamine-RuII catalysts and their representative reactions.
| Entry | Catalyst | Substrate | Product | S/C [a]; Time; Temp.; Conversion (%) |
% ee (config) [b] |
Ref. [c] |
|---|---|---|---|---|---|---|
| 1 | (R,R)-1a | 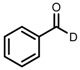 |
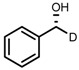 |
200; 0.5 h; 28 °C; 100 | 97 (R) | [26] |
| 2 | (S,S)-1b | 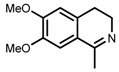 |
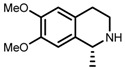 |
200; 3 h; 28 °C; 99 | 95 (R) | [53] |
| 3 | (R,R)-1b [d] | 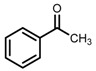 |
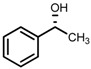 |
100; 20 h; 28 °C; > 99 | 97.7 (R) | [57] |
| 4 | (S,S)-1c | 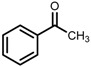 |
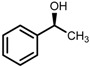 |
200; 20 h; 28 °C; 99 | 98 (S) | [54] |
| 5 | (S,S)-1c [d] | 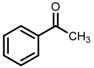 |
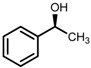 |
200; 15 h; r.t.; 99 | 97 (S) | [52] |
| 6 | (R,R)-1d | 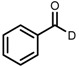 |
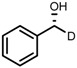 |
200; 7 h; 28 °C; 99 | 96 (R) | [26] |
| 7 | (S,S)-1e | 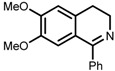 |
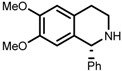 |
200; 8 h; 28 °C; 99 | 84 (R) | [53] |
| 8 | (R,R)-1f | 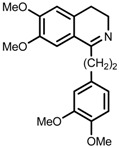 |
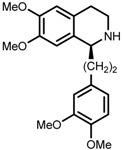 |
200; 12 h; 28 °C; 99 | 92 (S) | [53] |
[a] Molar ratio substrate/catalyst; [b] See corresponding references for details on determination of ee and product configuration; [c] Reference containing data for a given entry; [d] Catalyst was formed in situ from the [RuCl2(η6-arene)]2 dimer and the corresponding N-arylsulfonylethylene-1,2-diamine.
