Table 1.
In vitro antifungal activity of miconazole and analogues [2].
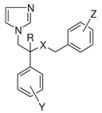 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| R | X | Y | Z | C.a.(a) | M.c. | T.m. | T.r. | A.f.(b) | |
| 2a | H | 0 | 2,4-Cl2 | 2,4-Cl2 | 10 | <1 | 0.1 | <1 | 10 |
| 2b | H | 0 | 2,4-Cl2 | 2,6-Cl2 | 100 | <1 | 0.1 | 0.1 | 10 |
| 2c | H | 0 | 4-F | 2,4-Cl2 | 100 | 10 | <1 | <1 | 10 |
| 2d | H | 0 | 2,4-Cl2 | 4-F | 100 | 10 | 10 | 10 | 10 |
| 2e | H | 0 | 2,4-Cl2 | 2-F | 100 | 10 | 10 | 10 | 100 |
| 2f | H | 0 | 2,4-Cl2 | 2-Cl | 100 | 10 | 0.1 | 0.1 | 10 |
| 2g | H | 0 | 4-Cl | 2,6-Cl2 | 10 | 100 | 10 | 10 | 100 |
| 2h | H | 0 | 2,4-Cl2 | 4-Cl | 100 | 0.1 | 0.01 | 0.1 | <1 |
| 2i | H | 0 | 4-Cl | 2,4-Cl2 | 10 | 10 | <1 | 0.1 | 10 |
| 2j | H | 0 | 2-Cl | 2,4-Cl2 | 100 | <1 | <1 | <1 | 10 |
| 2k | H | 0 | 4-Br | 2-Cl | 100 | 10 | 10 | 10 | 100 |
| 2l | H | 0 | 4-Br | 4-Cl | 10 | <1 | 0.1 | 0.1 | <1 |
| 2m | H | 0 | 4-F | 4-Cl | 100 | <1 | <1 | <1 | 10 |
| 2n | H | 0 | 4-F | 2-Cl | 100 | 100 | 10 | <1 | 100 |
| 2o | H | 0 | 4-Cl | 2-Cl | 100 | 100 | 10 | 10 | 100 |
| 2p | H | 0 | 4-Cl | 4-Cl | 10 | <1 | 0.1 | 0.1 | 10 |
| 2q | H | 0 | H | 4-Cl | 100 | 10 | <1 | <1 | 10 |
| 2r | H | 0 | H | H | >100 | 100 | 10 | 10 | 100 |
| 2s | H | 0 | 4-Me | 2,4-Cl2 | 100 | 100 | 10 | 10 | 100 |
| 2t | H | 0 | 2-Me | 2,4-Cl2 | 100 | 10 | 10 | <1 | 10 |
| 2u | H | 0 | 2,4-Cl2 | 2-Me | 100 | 10 | 0.1 | 0.1 | 10 |
| 2v | H | 0 | 2-Me | 2,4-Cl2 | 100 | <1 | <1 | <1 | <1 |
| 2w | H | 0 | 2,4-Cl2 | 3-MeO | 100 | 10 | 10 | 10 | 100 |
| 2x | H | 0 | 2,4-Cl2 | 4-MeO | 100 | 10 | 10 | 10 | 100 |
| 2y | H | 0 | 4-Cl | 2-Me | 100 | 100 | 10 | 10 | 100 |
| 2z | H | 0 | 4-Me | 2-Cl | 100 | 100 | 10 | 10 | 100 |
| 2aa | H | 0 | 4-Me | 4-Cl | 100 | 10 | <1 | <1 | 10 |
| 3a | Me | 0 | 4-Cl | 2,4-Cl2 | >100 | 10 | 10 | 10 | 100 |
| 3b | Me | 0 | H | 2,4-Cl2 | >100 | 100 | 10 | 10 | >100 |
| 3c | Me | 0 | 4-Cl | 4-Cl | 100 | 100 | 10 | 10 | 100 |
| 5 | H | NH | 4-Cl | 2-Cl | >100 | 100 | 100 | 100 | ND(c) |
| tolnaftate | >100 | >100 | 10 | <1 | >100 | ||||
| nystatin | 333 | ND | ND | ND | ND | ||||
(a) Lowest concentration of total inhibition (μg/mL); (b) C.a.: Candida albicans; M.c.: Microsporum canis; T.m.: Trichophyton mentagrophytes;T.r.: Trichophyton rubrum; A.f.: Aspergillus fumigates
(b) Figures proceeded by "<" represent the lowest dose level tested (µg/mL) with complete inhibition; (c) Figures proceeded by ">" denoted partial growth at 100 µg/mL; 2a: miconazole; 2b: isoconazole; 2h: econazole. (c) ND: not done.
