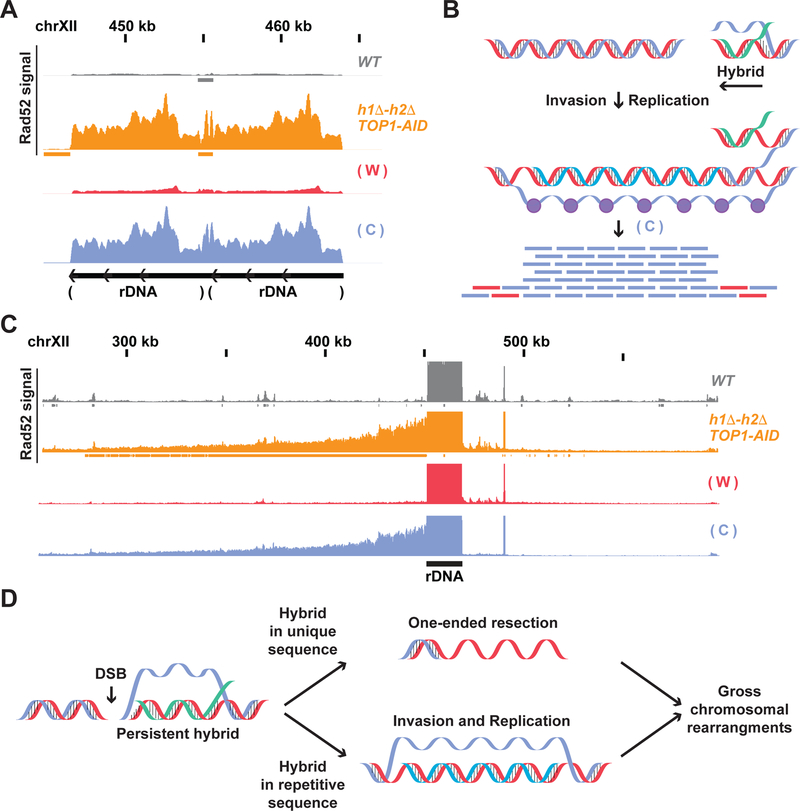
Fig. 7. Hybrids persistence induces different types of aberrant DNA structures.
(A) Rad52-ChIPseq signal from WT (gray) and h1Δ-h2Δ TOP1-AID (orange) strains treated with auxin to deplete Top1-AID on the rDNA locus. The strand signal in the h1Δ-h2Δ TOP1-AID strain is showed with a red bar-chart for the Watson strand, and blue for the Crick strand. The signal from the rDNA is originates mainly from the Crick strand. (B) Model for hybrid-induced damage in h1Δ-h2Δ TOP1-AID. When Top1 is depleted with auxin in the hΔ-h2Δ TOP1-AID strain, a DSB is induced at the 3’of the hybrid-RNA. The ssDNA from the R-loop (or the hybrid-RNA) triggers origin-independent DNA replication. The aberrant replication fork replicates only the Watson strand leaving a single stranded Crick coated by Rad52. (C) Rad52 signal from strains as in (A) from the unique region next to the rDNA. The signal from the unique sequence on the centromere-side of the rDNA locus is originated mainly from the Crick strand. (D) Model for hybrid-induced DNA damage. Hybrid persistence drives different aberrant DNA structure formation: persistent hybrids at unique sequences result in tens of kilobases of one-ended resected DNA, while hybrids at highly repetitive sequence drive origin-independent replication that proceeds for hundreds of kilobases. These aberrant ssDNA structures can be precursors of gross chromosomal rearrangements like deletions and duplications.