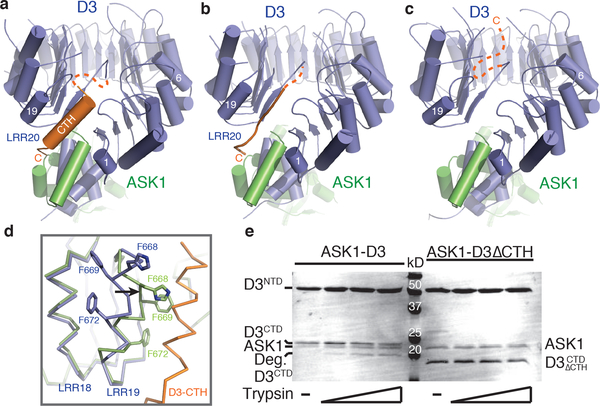
Figure 1. Structural plasticity of the D3 C-terminal 𝛂-helix.
(a-c) Overall structures of ASK1 (green) bound to D3 (blue) with its C-terminal α-helix in variable conformation. (d) Superposition of ASK1-D3 structures shown in (a) (blue) and (c) (green) focusing on LRR18–20. LRR20 (orange) is disordered in the third crystal form (c). Black arrow indicates the conformational shift of LRR19 when LRR20 is disordered. (e) Limited proteolysis of ASK1-D3 complex ± CTH (repeated thrice). The D3 protein was purified with its N-terminal and C-terminal segments (NTD & CTD) tightly associated (see Methods).