Abstract
Tongue is one of the most common subsites involved by oral cancer. Improved surgical care and adjuvant therapy, along with better rehabilitation has significantly improved long-term survival and quality of life in patients with oral cancer. Primary surgical treatment is the preferred modality of treatment in cancers of the oral tongue. Although the surgical techniques have remained unchanged, various devices have been used to aid primary tumour resection, with a view to reduce bleeding and scarring. The purpose of this study is to compare resection of oral tongue tumours using ultrasonic coagulation device with conventional electrodiathermy. This study was conducted to compare histological margins and duration of surgery between ultrasonic coagulation device and electrodiathermy in the resection of oral tongue tumours. A retrospective analysis was performed comprising patients undergoing wide excision for squamous cell carcinoma of the lateral tongue, with either ultrasonic coagulation device or conventional electrodiathermy at Our Institute in Mumbai, India, from October 2015 to December 2016. Single factor ANOVA with the level of significance set at 95% and alpha value of 0.05. Patients who underwent excision with ultrasonic coagulation device better histologically tumour-free margins (except posterior margin) (p values—anterior margin, posterior margin, lateral margin and deep margin 0.0045, 0.59, 0.011 and 0.00013 respectively) and lesser operative time when compared with conventional electrodiathermy. Ultrasonic coagulation device was effective in providing adequate oncologically safe margins in carcinoma tongue.
Keywords: Electrodiathermy, Tongue cancer, Tumour margin, Ultrasonic coagulation device
Introduction
Oral squamous cell carcinoma accounts for over 200,000 newly diagnosed cases of cancer worldwide annually. In India, it accounts for almost a third of the cancer burden [1, 2]. Primary surgical treatment is recommended for carcinoma of the oral tongue [3] and various surgical aids have been used to perform the primary tumour resection. These include cold steel scalpel, cold steel scissors, monopolar and bipolar electrodiathermy, laser (carbon dioxide, argon laser, diode laser) and ultrasonic coagulation devices. Each of these aids has its merits and demerits; very few studies have compared their use in oral cancer resections.
Although there have been studies on parameters like blood loss, post-operative pain and wound healing in oral tongue cancer operated with Ultrasonic Coagulation Device [4], very little data exists on the status of margins, which is arguably one of the most important predictors of outcome following surgery [5].
The purpose of this study was to compare histological margin status and duration of surgery in patients treated with ultrasonic coagulation device and electrodiathermy for the resection of oral tongue tumours.
Subjects and Methods
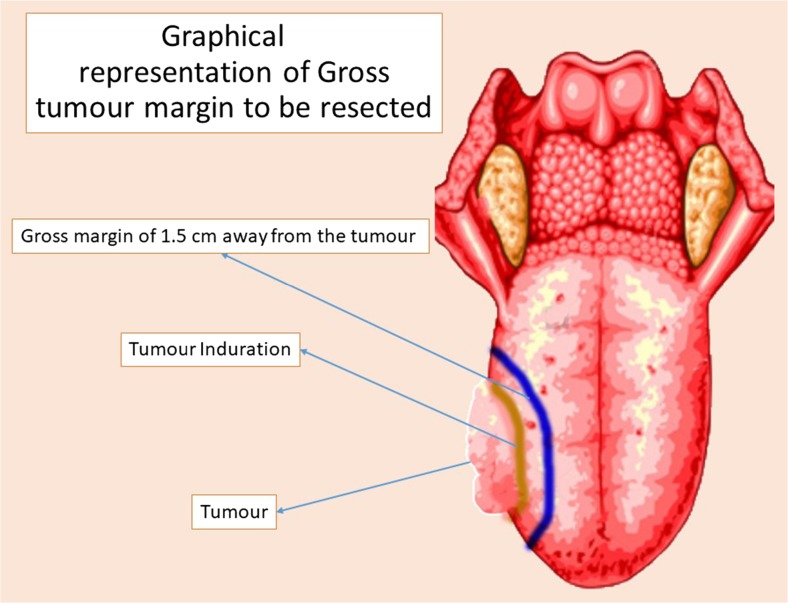
A retrospective analysis was performed comprising patients undergoing wide excision for squamous cell carcinoma of the lateral tongue, with either ultrasonic coagulation device or conventional electrodiathermy at our institute in Mumbai, India, from October 2015 to December 2016. Twenty-four patients of previously untreated squamous cell carcinoma of the oral tongue (T: stages 1 and 2) were included in the study, who all underwent wide excision of tongue and neck dissection. A gross margin of 1.5 cm away from the tumour (Fig. 1) was the intention in all resections. Informed consent was obtained from all individual participants included in the study.
Fig. 1.
Graphical representation of gross tumour margin to be resected
Twelve patients of group 1 (50%) underwent excision with monopolar electrodiathermy (KLS Martin Maximum © Gebrüder Martin GmbH & Co. KG, Germany)—tongue was resected with a coagulation current using monopolar electrodiathermy adjusted at 40 W power using a standard tip. Twelve patients in group 2 (50%) underwent the excision with ultrasonic coagulation device (Olympus – Thunderbeat, Japan). The tongue was held at the mucosa incision line between the jaws of the handpiece (Model - TB-0520IC: 20 cm with incline grip) and power was at level 2.
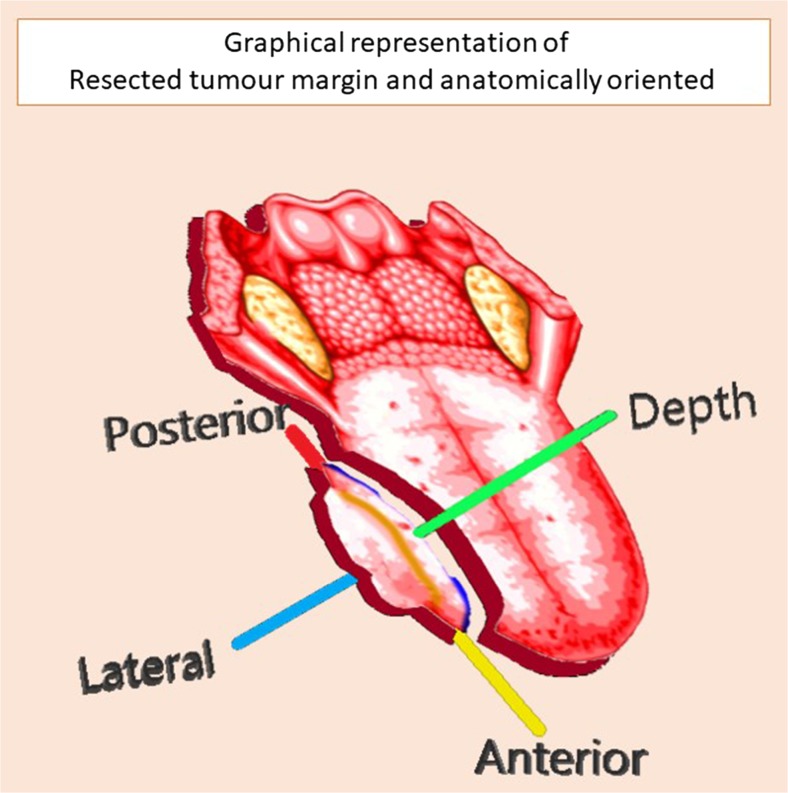
The excised specimens were tagged (Fig. 2) at the cut margins, oriented, fixed in formalin 10% and were sent for histopathological examination at the earliest. The time required for excision of primary tumour was calculated from the time of incision till removal of the specimen.
Fig. 2.
Graphical representation of resected tumour margin and anatomically oriented, depicting anterior, lateral, deep and posterior margin
Statistical Analysis
Descriptive statistics were used to evaluate study information. The association was analysed by the single factor ANOVA with the level of significance set at 95% and alpha value of 0.05.
These two groups were compared for differences in:
Histopathological margins
Duration of surgical excision
IBM SPSS software version 24.0 was used for all statistical evaluations.
Results
A total of 24 patients were studied. Of these patients, 18 were male (75%) and 6 were female (25%). Median age of 51.58 years. Seventeen patients (70.8%) had well-differentiated squamous cell carcinoma and 7 patients (29.2%) had moderately differentiated squamous cell carcinoma.
Group 1 (group undergoing excision with conventional electrodiathermy) composed of 12 patients (50%), 3 female (25%) and 9 males (75%)
Group 2 (group undergoing excision with ultrasonic coagulation device) composed of 12 patients (50%), 3 females (25%) and 9 males (75%)
A statistically significant improvement in anterior, lateral and deep resected margins are observed with group 2 (Table 1). An improvement in the duration for primary tumour excision (Table 2) is also seen with group 2.
Table 1.
Resected margin comparison between the ultrasonic coagulation device and electrocautery
| Groups | Anterior margin | Posterior margin | Lateral margin | Deep margin | |
|---|---|---|---|---|---|
| Ultrasonic coagulation device | Mean value (mm) | 11.91* | 10.58 | 10.33* | 11.33* |
| Standard deviation | 1.78 | 1.56 | 1.07 | 1.49 | |
| Variance | 3.17 | 2.44 | 1.15 | 2.24 | |
| Electrocautery | Mean value (mm) | 10.17 | 10.25 | 9.33 | 9.08 |
| Standard deviation | 0.718 | 1.422 | 0.65 | 0.79 | |
| Variance | 0.515 | 2.023 | 0.42 | 0.63 | |
| p value | 0.0045* | 0.59 | 0.011* | 0.00013* | |
*Statistically significant
Table 2.
Time duration comparison between the ultrasonic coagulation device and electrocautery
| Groups | Median value (min) | Standard deviation | Variance | p value |
|---|---|---|---|---|
| Ultrasonic coagulation device | 14.58* | 3.96 | 15.71 | 5.635 × 10−8 |
| Electrocautery | 25.62 | 3.20 | 10.26 |
*Statistically significant
Discussion
One of the new entrants to the electrosurgical unit is the ultrasonic coagulation device. This device dissects by using ultrasonic vibrations of around 55,000 Hz transmitted between the instrument blades. The active blade of the instrument vibrates longitudinally against an inactive blade over an excursion of 50 to 100 mm by disrupting tertiary hydrogen bonds within the tissue [6]. This takes place at a relatively low temperature (80 °C), causing less tissue injury than the other adopted techniques [7, 8]. Given that the water part of the tissue does not reach the boiling point, the relatively mild increase in temperature causes the proteoglycans and the collagen fibres in the tissues to become denatured and mix with intracellular and interstitial fluids to form a glue like substance (a coagulum) [9]. The generation of local water vapour was questioned for triggering the release of cancer cells. However, it is demonstrated by animal studies that viable air-borne cancer cells are not released by using an Ultrasonic Coagulation Device [10].
The shaft of the instrument remains relatively cool and there is no electrical conduction through the handpiece; this may reduce the risk of inadvertent injury to surrounding structures such as the lingual nerve or the hypoglossal nerve or the normal mucosal margin at the time of the hemiglossectomy.
The benefits of simultaneous haemostasis and cutting would facilitate the surgery without interruption for haemostasis even with clear margins and a bloodless field. The most critical aspect in surgical resection is deep and posterior margins, especially for deep infiltrations or an ill-defined tumour edge with islands of tumour cells in advance of clinical tumour edge [11].
In contrast, monopolar electrodiathermy works by alternating electrical current with various frequencies applied to the patient’s tissue. Electric charge is absorbed by molecules in the tissue, leading to tissue heating from 300 to 400 °C. This results in disruption by either boiling or coagulating, depending on the settings and application of the device. This heat-based mechanism of action is responsible for the comparatively large zone of tissue injury adjacent to the surgical incision [4].
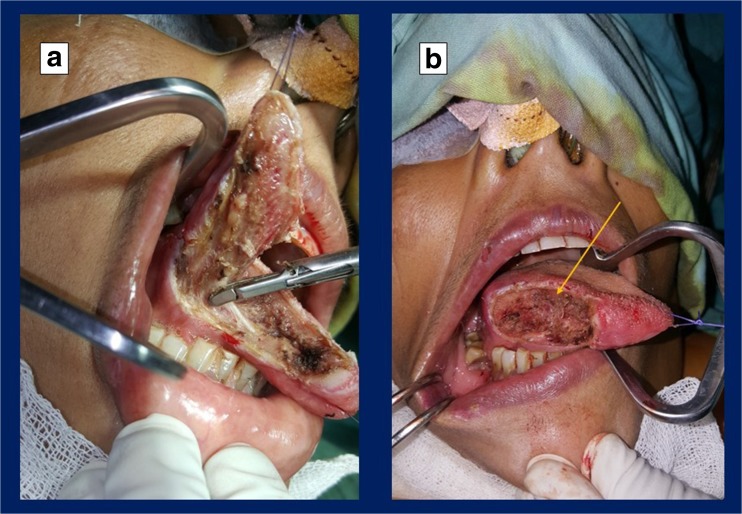
Patients who underwent resection with ultrasound coagulation device in our study had wider margins and shorter duration of surgery when compared with conventional electrodiathermy. This may be due to the reduced zone of tissue trauma and clearer assessment of the tumour-free margin (Fig. 3). There were no significant differences in resected posterior margins between the ultrasonic coagulation device and the electrodiathermy, possibly because of the anatomical hindrance to reach the posterior aspect of tongue tumours with the intraoral approach.
Fig. 3.
a Depicting the wide local excision of the right-sided tongue tumour with ultrasonic coagulation device. b Post-tumour excision defect in the same patient
According to histological data by Cristalli et al. [12], the artefact depth was present with a mean of 0.8 mm in the electrodiathermy specimen group and 0.4 mm in the harmonic shears group. There were less cautery/crush artefacts and extravascular blood clots observed in the harmonic shears group. This data gives a critical insight into possible over or under treatment of margins, if the artefacts are not considered in the pathology reporting.
The surgical resections were performed significantly sooner with ultrasonic coagulation device in comparison with electrodiathermy. Simultaneous haemostasis and cutting is achieved with ultrasonic coagulation device explaining the reduction in duration for the primary tumour excision.
Ultrasonic coagulation devices are not without drawbacks—they are bulky devices, which may pose problems with compromised mouth opening or a posteriorly placed tumour, are expensive, have a blunt cutting edge and a learning curve associated with their use.
Further potential areas of study included should evaluate the assessment of post-operative pain after surgery and healing.
Conclusion
Ultrasonic coagulation device was associated with improved margins and reduced operative time in resection of oral tongue carcinoma when compared with conventional electrodiathermy.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Karthik N. Rao, Phone: +91-8861373839, Email: karthik.nag.rao@gmail.com
Mohan Jagade, Email: mohanjagade@gmail.com.
Vitthal D. Kale, Email: drvdkale@yahoo.com
Devkumar Rengaraja, Email: devkumar16@gmail.com.
Amol Hekare, Email: amolhekare15@gmail.com.
References
- 1.Sankaranarayanan R, Ramadas K, Thomas G, Muwonge R, Thara S, Mathew B, et al. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarani MR. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4:29–35. doi: 10.5005/jp-journals-10001-1132. [DOI] [Google Scholar]
- 3.National Comprehensive Cancer Network . NCCN guidelines and clinical resources. Fort Washington: National Comprehensive Cancer Network; 2011. [Google Scholar]
- 4.Metternich FU, Wenzel S, Sagowski C, Ja kel T, Koch U. The “Ultracision Harmonic Scalpel” ultrasound activated scalpel: initial results in surgery of the tongue and soft palate. HNO. 2002;50:733–738. doi: 10.1007/s00106-001-0596-2. [DOI] [PubMed] [Google Scholar]
- 5.Bernier J, Cooper J, Pajak T, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 6.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue: prognostic and therapeutic implications. Am J Surg. 1986;152:354–360. doi: 10.1016/0002-9610(86)90304-1. [DOI] [PubMed] [Google Scholar]
- 7.Brandwein-Gensler M, Teixeira MS, Lewis CM. Oral squa- mous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 8.Sparano A, Weinstein G, Chailian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131:472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Belli G, Fantini C, Ciciliano F, et al. Pancreaticoduodenectomy in portal hypertension: use of the Ligasure. J Hepato-Biliary-Pancreat Surg. 2003;10:215–217. doi: 10.1007/s00534-002-0745-3. [DOI] [PubMed] [Google Scholar]
- 10.Ou CS, Harper A, Liu YH, Rowbotham R. Laparoscopic myomectomy technique: use of colpotomy and the harmonic scalpel. J Reprod Med. 2002;47:849–853. [PubMed] [Google Scholar]
- 11.Palazzo FF, Francis DL, Clifton MA. Randomized clinical trial of Ligasure versus open haemorrhoidectomy. Br J Surg. 2002;89:154–157. doi: 10.1046/j.1365-2168.2002.01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Cristalli G, Mercante G, Covello R, Sperduti I, Cristalli MP, Spriano G. Histopathological assessment in glossectomy: harmonic shears versus monopolar electrosurgery pilot study. Otolaryngol Head Neck Surg. 2012;147(6):1076–1082. doi: 10.1177/0194599812456966. [DOI] [PubMed] [Google Scholar]