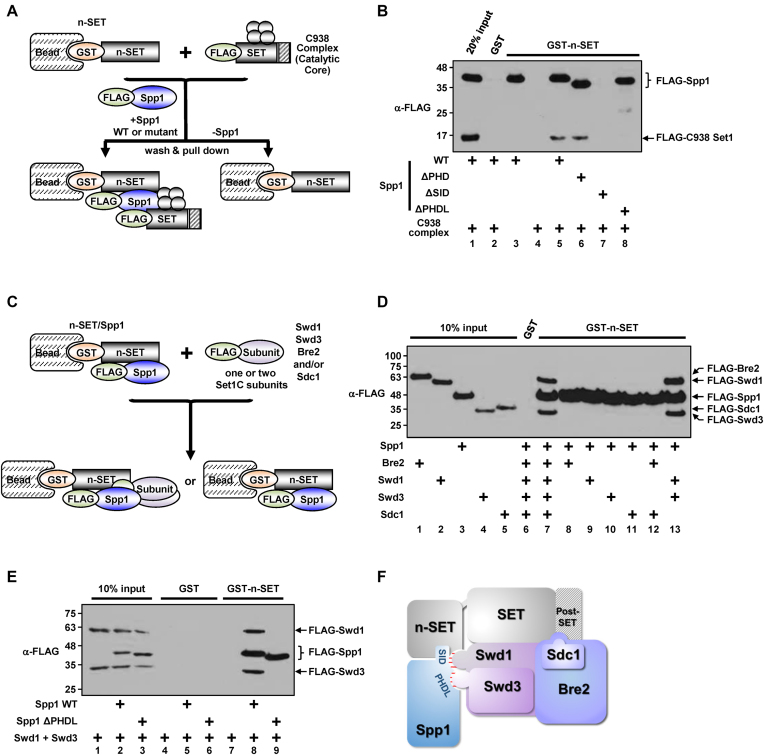
Figure 2.
Direct domain-subunit interactions within the C762 Set1 complex. (A) A Schematic representation of the in vitro protein interaction assays in (B). Four gray-colored circles indicate Swd1, Swd3, Bre2 and Sdc1. (B) Requirement of the SID and PHDL domains of Spp1 for trans-interaction between the n-SET domain and the catalytic core of Set1C. The purified C938 complex (Supplementary Figure S2A) was tested for binding to GST-n-SET in the presence of purified WT or mutant Spp1. (C) A Schematic representation of the in vitro protein interaction assays in (D) and (E). (D) Physical interaction of Swd1 and Swd3 with Spp1 bound to the n-SET domain. Combinations of purified Set1C subunits (Supplementary Figure S2B) as indicated were tested for binding to GST-n-SET in the presence of purified Spp1. Note that FLAG-Sdc1 ran slower than FLAG-Swd3 in this assay where a gradient SDS-PAGE gel was used for better resolution of protein migrations. (E) Requirement of the PHDL domain for Spp1-mediated interaction of the n-SET domain with Swd1/Swd3 heterodimer. Purified Swd1 and Swd3 proteins were tested for binding to GST-n-SET in the presence of either purified WT or ΔPHDL-Spp1. (F) A schematic model of subunit/domain interactions in the C762 Set1 complex. A surface created by the n-SET domain and Spp1 interacts with heterodimeric Swd1/Swd3 (highlighted by red lines). Direct association of heterodimeric Swd1/Swd3 and Bre2/Sdc1 with SET and post-SET domains has been established by previous studies (15,33,34). The SID domain may also contribute to the proper subunit-interaction network in the catalytic region of Set1C, as inferred from the selective loss of H3K4me3 in the ΔSID-Spp1–containing complex (Figure 1G and H).