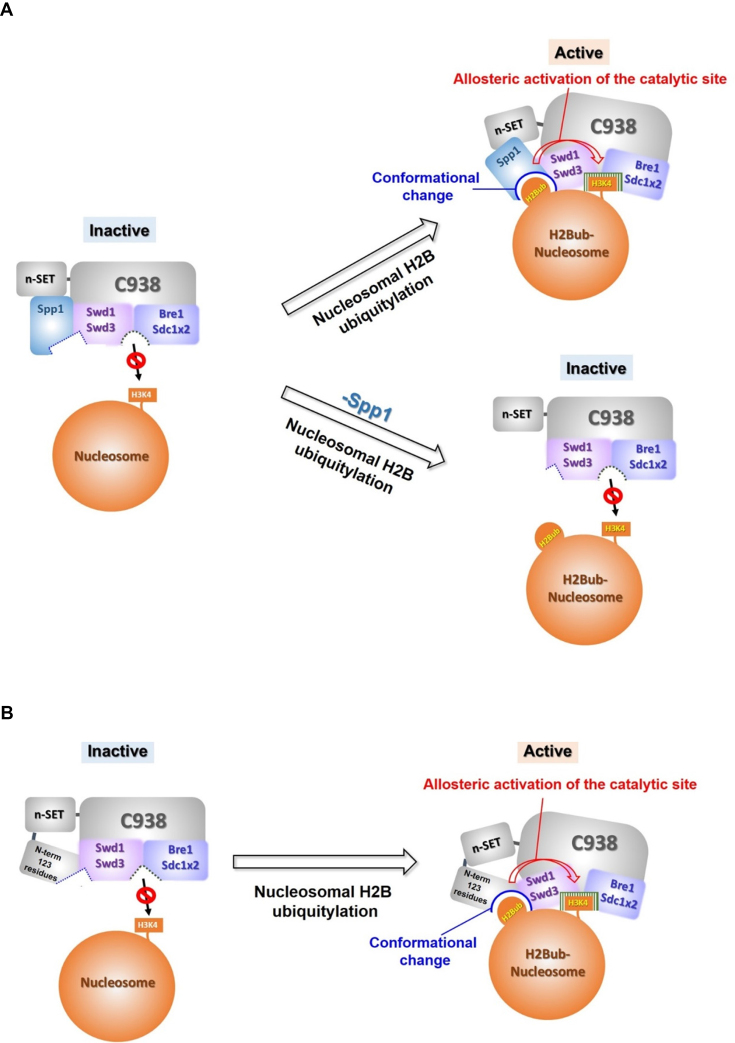
Figure 7.
Mechanistic models of H2B ubiquitylation-dependent H3K4 methylation involving Spp1 and the Set1 N-terminal region. (A) In the absence of the Set1 N-terminal region, we envision that H2B ubiquitylation causes conformational changes in the catalytic region of Set1C that involve rearrangements of the subunit-interaction network; this, in turn, induces allosteric activation of the catalytic site, rendering it accessible to nucleosomal H3K4. This conformational change does not occur in the absence of Spp1, as evidenced by the fact that Spp1 is essential for the interaction between n-SET/Spp1 and Swd1/Swd3, which is involved in sensing H2Bub and/or conformational changes. (B) In the absence of Spp1, we envision that basic motifs within the Set1 N-terminal region physically interact with Swd1, creating a surface for sensing H2Bub in conjunction with other subunits in the catalytic region of Set1C. This also induces allosteric activation of the catalytic site of Set1C, rendering it favorable for nucleosomal H3K4 methylation. Sdc1 × 2 indicates the dimeric association of Sdc1 in the catalytic region, which was established in recent reports (33,34).