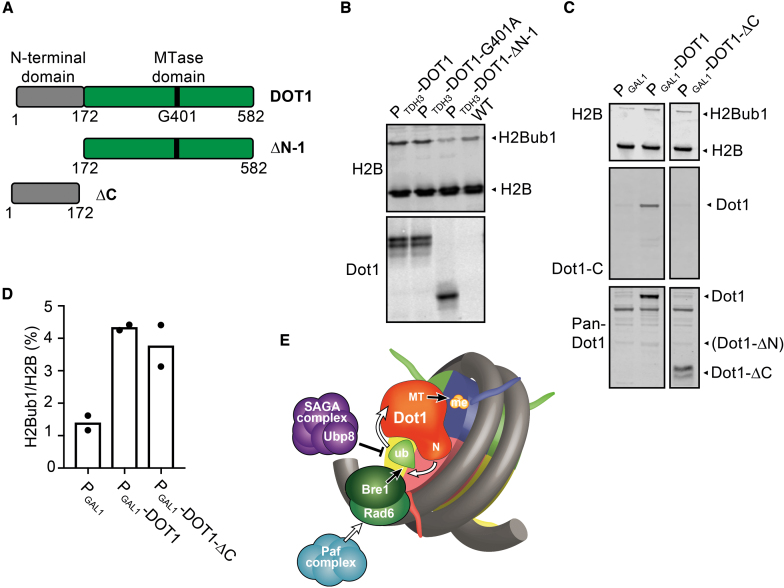
Figure 4.
The N-terminus of Dot1 is necessary and sufficient to promote H2Bub1. (A) Schematic representation of the yeast Dot1 protein and different mutants used in this study. (B) Deletion of the Dot1 N-terminal part (DOT1-ΔN-1) abolished the effect of Dot1 on H2Bub1 without altering the expression level of constitutively overexpressed Dot1. (C) Inducible overexpression in galactose media of the N-terminus alone (Dot1-ΔC) was sufficient to promote H2Bub1. The lane with DOT1-ΔC-terminal originates from the same blot. The pan-Dot1 antibody was raised against full-length Dot1, but preferentially recognizes the N-terminus (Supplementary Figure S4). (D) Quantification of the immunoblot shown in (C) (mean and individual data points of two biological replicates). (E) A model for the mutual crosstalk between Dot1 and H2Bub. The catalysis of (de)modifying reactions is indicated by a black arrow or bar-headed line. Stimulation is visualized by white arrows. In short, H2BK123ub1 promotes H3K79 methylation by Dot1 and the N-terminus of Dot1 promotes ubiquitination of H2BK123. The latter stimulation is independent of deubiquitination by Upb8 and the recruitment of Bre1/Rad6 by the Paf complex, and is thus likely to directly act on ubiquitination by Bre1.