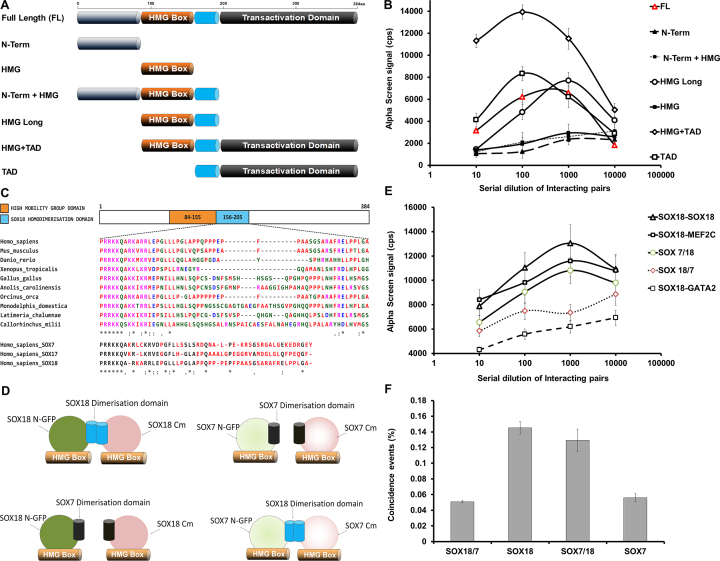
Figure 3.
Biochemical characterization of the dimerization domain of SOX18. (A) Schematic representation of 7 mutants representing domain specific truncations of SOX18. (B) Typical curves of AlphaScreen signal as a function of concentration for the seven SOX18 truncations. Only FL, HMG-TAD, HMG long and TAD form dimer whereas N-Term, N-Term-HMG and HMG show no signal. (C) Top: multiple sequence alignment of the SOX18 homodimerization domain across 10 different species, using the human SOX18 homodimer domain as a reference. Residues are grouped into colours, based on their chemical and physical properties. Bottom: multiple sequence alignment of the putative SOX18 dimerization domain with the corresponding domains in SOX17 and SOX7 proteins. The 50 amino acids directly following the high mobility group (HMG) domain of the SOXF protein family reveals residues that are non-conserved and therefore possibly involved in the unique ability for SOX18 to homodimerize. Residues that are not conserved between all SOXF family members are highlighted in red. For all, fully conserved residues are marked by an asterisk (*), partially conserved residues that retain high similarity are marked by a colon (:), partially conserved but weakly similar residues are marked by a full stop (.) and residues that have no conservation are left blank (). Protein alignment was performed using Clustal Omega. (D) Schematic representation of the constructs: (top) SOX18 WT and SOX7 WT; (bottom) SOX18DIM/SOX7 and SOX7DIM/SOX18 swaps. (E) Typical AlphaScreen curves obtained for SOX18 WT with MEF2C and SOX7DIM/SOX18 showing respectively a positive signal above 10 000 cps. Lack of signal for the SOX18DIM/SOX7 swap indicates a loss of the dimerization propensity. SOX18-GATA2 is used as a negative control. (F) Value of coincidence obtained from the two-colours coincidence experiments performed on SOX18 WT, SOX18/7, SOX7/18 and SOX7 WT co-expression as a GFP/Cherry pair. Data were analysed as in Figure 1 and the percentage of coincident events (0.25 < C < 0.75) was plotted for the different constructs, reflecting their ability to homodimerize.