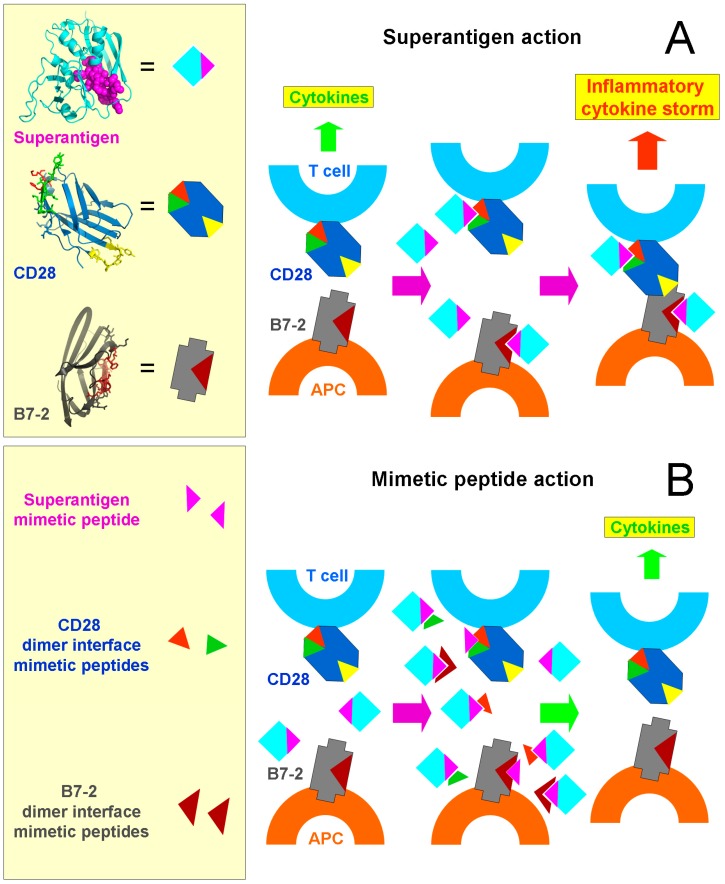
Figure 1.
Mechanism of action of superantigen toxins and of mimetic peptide antagonists. (A) The superantigen acts to induce an inflammatory cytokine storm. In the prototypical superantigen SEB, the conserved β-strand/hinge/α-helix superantigen domain (magenta) points away from the binding sites for MHC-II molecule and TCR that were omitted for clarity; the extracellular domain of CD28 shows the homodimer interface (red and green) and the site where its B7 coligands bind (yellow) and the extracellular domain of B7-2 shows the crystallographic homodimer interface (brown). Normal immune stimulation leads to a moderate CD28–B7-2 interaction between antigen-presenting cell and T cell and to moderate cytokine induction (left). However, when the superantigen engages the homodimer interface of CD28 and of B7-2 (middle), this potently enhances CD28–B7-2 engagement, resulting in an inflammatory cytokine storm (right) [8]. (B) Short peptide mimetics of the β-strand/hinge/α-helix superantigen domain [1] or of the homodimer interfaces in CD28 [7] or in B7-2 [8] act as competitors that prevent access of the superantigen to its CD28 and B7-2 host targets (middle) and thereby abrogate excessive signaling through the CD28/B7-2 axis (right).