Abstract
Background:
Over the past decade, prehospital and in-hospital treatment for out-of-hospital cardiac arrest (OHCA) has improved considerably. There are sparse data on the long-term outcome, especially in elderly patients. We studied whether elderly patients benefit to the same extent compared with younger patients and at long-term follow up as compared with the general population.
Methods:
Between 2001 and 2010, data from all patients presented to our hospital after OHCA were recorded. Elderly patients (⩾75 years) were compared with younger patients. Neurological outcome was classified as cerebral performance category (CPC) at hospital discharge and long-term survival was compared with younger patients and predicted survival rates of the general population.
Results:
Of the 810 patients admitted after OHCA, a total of 551 patients (68%) achieved return of spontaneous circulation, including 125 (23%) elderly patients with a mean age of 81 ± 5 years. In-hospital survival was lower in elderly patients compared with younger patients with rates of 33% versus 57% (p < 0.001). A CPC of 1 was present in 73% of the elderly patients versus 86% of the younger patients (p = 0.031). In 7.3% of the elderly patients, a CPC >2 was observed versus 2.5% of their younger counterparts (p = 0.103). Elderly patients had a median survival of 6.5 [95% confidence interval (CI) 2.0–7.9] years compared with 7.7 (95% CI 7.5–7.9) years of the general population (p = 0.019).
Conclusions:
The survival rate after OHCA in elderly patients is approximately half that of younger patients. Elderly patients who survive to discharge frequently have favorable neurological outcomes and a long-term survival that approximates that of the general population.
Keywords: cardiopulmonary resuscitation, cerebral performance, elderly, long-term mortality, out-of-hospital cardiac arrest, prognosis
Introduction
Outcome after out-of-hospital cardiac arrest (OHCA) has markedly improved in the past years.1–3 While the overall mortality rate is still considerable, the patients who do survive tend to have a good neurological outcome.4–6 Many studies have focused on short-term outcome after OHCA,7–11 and studies on the elderly have increased during the past years.12–18 Since older studies reported dismal outcomes in elderly patients after cardiopulmonary resuscitation (CPR),19 there still may be reluctance to institute maximal treatment in all patients for fear of only generating a greater number of survivors with poor functional outcomes.20,21
Several recent studies show that with increasing age, short-term survival decreases but survivors display a good neurologic outcome.13–15 Long-term survival after OHCA seems to approach the general population,22–25 but little is known on the long-term survival of elderly patients who survive until hospital discharge.16–18 To what extent elderly OHCA patients benefit from early treatment as compared with younger patients and the general population was investigated in the current study.
Materials and methods
Study design and setting
The University Medical Center Groningen (UMCG) is the main tertiary referral hospital for the north-eastern part of the Netherlands, which provides 24/7 emergency care in a region with 750,000 inhabitants.26 It operates in combination with multiple emergency medical services (EMS) in this area. A driver with a paramedical background and a nurse staff each ambulance, and both are trained and qualified to perform Advanced Cardiac Life Support (ACLS) according to the European Resuscitation Council guidelines, such as advanced airway interventions, administration of inotropic drugs and use of a defibrillator.27 In case of resuscitation, a backup ambulance is always sent. The nurse staff always commences CPR but has the discretion to discontinue if the patient has a ‘do not attempt CPR’ decision or a nonshockable rhythm present for more than 20 minutes. Except for these patients, all other OHCAs including EMS-witnessed arrests are transported to our center where resuscitation is continued or postresuscitation care is given following ACLS guidelines.27 When patients present with an ST-segment elevation myocardial infarction (STEMI) after OHCA we perform percutaneous coronary intervention (PCI) in patients with return of spontaneous circulation (ROSC), including elderly patients.28 Patients requiring continuous mechanical ventilation were transferred to the intensive care unit (ICU) for postresuscitation care. Treatment was left at the discretion of the attending physicians. If brainstem reflexes (i.e. absent pupillary light response and corneal reflexes) and motor scores were absent, no further life-sustaining treatment was delivered. In all other cases, additional somatosensory-evoked-potential (SSEP) monitoring was done and in the case of absent bilateral negative 20 (N20) SSEPs, active treatment was also withdrawn. Electroencephalography (EEG) analysis was performed when seizures or myoclonus was present. Seizures were treated with antiepileptic drugs; in the case of myoclonus status epilepticus, treatment was also stopped.29,30 In these patients, active therapies were discontinued, and treatment was altered to comfort care. The institutional review board [Medisch Ethische Toetsingscommissie (METc) of the UMCG] approved the study and waived the need for informed consent due to the observational nature of the study (METc 2011.374).
Patients
We studied all adult (⩾ 18 years) patients who were transferred to our hospital after OHCA during the years 2001–2010. Data were collected retrospectively from the medical records of the Ambulance System. During the study period, mild therapeutic hypothermia (MTH) was initiated if patients had a Glasgow Coma Score < 8. These patients were cooled to 32–34°C for 24 h and thereafter, passively rewarmed to 36°C. In this cohort, we defined patients with an age of ⩾ 75 years as elderly and used the younger patients for comparison of outcomes in both categories.
Data on prehospital characteristics, medical history, electrocardiography (ECG), SSEP, EEG and laboratory values were gathered from the hospital information system. Neurological status was classified using the cerebral performance category (CPC) from the clinical patient notes made during outpatient follow-up assessments by cardiologists, rehabilitation specialists or, when indicated, neurologists. Survival status of patients discharged alive was obtained from the municipal personal records database on 20 December 2017, with a maximum follow up of 16.4 years.
Statistical analysis
Continuous data are presented as either means ± standard deviation for normally distributed variables or medians and interquartile ranges for skewed data, or as group percentages with 95% confidence intervals (CI) for categorical variables. We determined outcome in subgroups dependent on initial rhythm [ventricular fibrillation (VF) versus no-VF] and implemented treatment (PCI versus no PCI, MTH versus no MTH). We recorded age, sex and known factors associated with outcome: initial rhythm and in-hospital treatment (PCI and MTH) and left ventricular function.
Statistical analysis was performed using the chi-square test for categorical and the Mann–Whitney U test or Student’s t test for skewed continuous and normally distributed variables, respectively. Kaplan–Meier survival analysis was used to assess long-term survival and the log-rank test was used to compare survival across subgroups. For each OHCA patient, the actual survival was compared with the predicted survival rates of the general population standardized for age, sex and calendar year. These predicted survival rates were obtained from the Dutch Census Bureau, which predicts general population survival annually.31 We identified independent predictors of in-hospital mortality, coronary angiography (CAG), PCI and MTH using multivariable logistic regression analyses. Univariable analyses were conducted on all known predictors for mortality, which were added without any transformation. A p < 0.25 was used for inclusion in the multivariable model. Calibration of the multivariable models was checked with Hosmer–Lemeshow tests and by plotting observed proportions against the predicted risks of 10 equally sized groups. We used complete-case analyses because all variables in our multivariable models had fewer than 5% missing values.32 Statistical significance was defined as a two-sided p value of < 0.05. Statistical analyses were performed with Stata version 15.1 (StataCorp, 2017, College Station, Texas: StataCorp LLC).
Results
Prehospital characteristics of all admitted patients
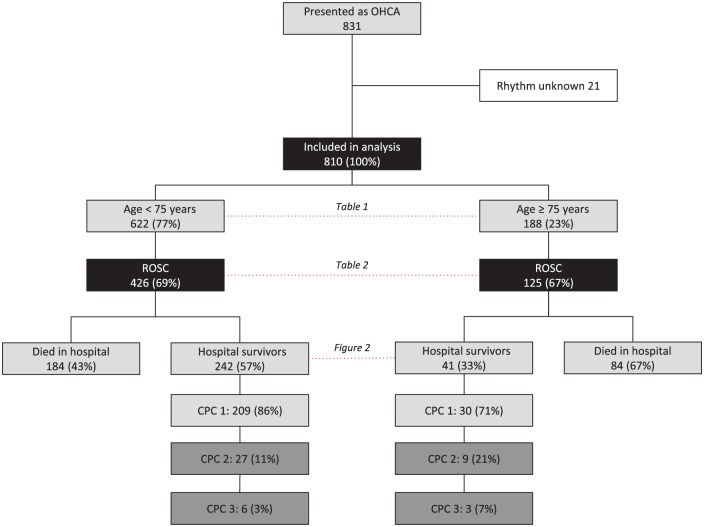
From 2001 to 2010, a total of 810 patients were admitted after OHCA (Figure 1). Of these, 188 (23%) patients were ⩾ 75 years with a mean age of 81 ± 5 years. Baseline and clinical characteristics of patients aged < 75 years and ⩾ 75 years are presented in Table 1. The location of arrest differed between both groups and elderly patients less frequently had an initial rhythm of VF. There was no difference between the two age groups in the fraction of patients who achieved ROSC, although upon arrival at the emergency department, younger patients more often had achieved ROSC. Elderly patients also had an increased incidence of prior cardiovascular disease and risk factors. More specifically, elderly patients were more frequently known with a previous myocardial infarction, cerebrovascular accident, hypertension, diabetes and concurrent malignancy.
Figure 1.
Flow diagram of study population.
CPC, cerebral performance category; OHCA, out-of-hospital cardiac arrest; ROSC, return of spontaneous circulation.
Table 1.
Prehospital characteristics and medical history of all patients.
| Total n = 810 |
< 75 years n = 622 |
⩾ 75 years n = 188 |
p value | |
|---|---|---|---|---|
| Age* | 63 ± 15 | 57 ± 12 | 81 ± 5 | <0.001 |
| Male sex (%) | 572 (71%) | 448 (72%) | 124 (66%) | 0.110 |
| Prehospital characteristics | ||||
| Location (%) | <0.001 | |||
| Home | 374 (46%) | 280 (45%) | 94 (50%) | |
| Public place | 190 (24%) | 164 (26%) | 26 (14%) | |
| Other | 116 (14%) | 91 (15%) | 25 (13%) | |
| Ambulance | 60 (7%) | 47 (8%) | 13 (7%) | |
| Unknown | 70 (9%) | 40 (6%) | 30 (16%) | |
| Witnessed arrest (%) | 642 (79%) | 504 (81%) | 138 (73%) | 0.964 |
| Bystander CPR (%) | 460 (57%) | 366 (59%) | 94 (50%) | 0.160 |
| Initial rhythm (%) | <0.001 | |||
| VF | 518 (64%) | 421 (68%) | 97 (52%) | |
| PEA | 149 (18%) | 97 (16%) | 52 (28%) | |
| Asystole | 116 (14%) | 84 (14%) | 32 (17%) | |
| Pulseless VT | 27 (3%) | 20 (3%) | 7 (4%) | |
| ROSC before ED arrival (%) | 473 (58%) | 374 (60%) | 99 (53%) | 0.013 |
| ROSC (%) | 551 (68%) | 426 (69%) | 125 (67%) | 0.606 |
| Cardiovascular history and risk factors | ||||
| History of CVD (%) | 257 (32%) | 169 (27%) | 88 (48%) | <0.001 |
| Myocardial infarction (%) | 148 (18%) | 98 (16%) | 50 (27%) | <0.001 |
| STEMI (%) | 131 (16%) | 86 (14%) | 45 (24%) | <0.001 |
| PCI (%) | 61 (8%) | 46 (7%) | 15 (8%) | 0.680 |
| CABG (%) | 57 (7%) | 31 (5%) | 26 (14%) | <0.001 |
| Angina (%) | 79 (10%) | 49 (8%) | 30 (16%) | <0.001 |
| Cerebrovascular accident (%) | 51 (6%) | 33 (5%) | 18 (10%) | 0.024 |
| History of VF (%) | 16 (2%) | 11 (2%) | 5 (3%) | 0.400 |
| Malignancy (%) | 35 (4%) | 16 (4%) | 19 (10%) | <0.001 |
| Hypertension (%) | 196 (24%) | 141 (23%) | 55 (29%) | 0.031 |
| Hypercholesterolemia (%) | 78 (10%) | 65 (11%) | 13 (7%) | 0.180 |
| Diabetes (%) | 108 (13%) | 72 (12%) | 36 (19%) | 0.004 |
| Medication history | ||||
| Beta blocker (%) | 185 (23%) | 124 (20%) | 61 (32%) | <0.001 |
| ACE inhibitor (%) | 125 (15%) | 80 (13%) | 45 (24%) | <0.001 |
| AT-II receptor antagonist (%) | 56 (7%) | 39 (6%) | 17 (9%) | 0.043 |
| Diuretics (%) | 142 (18%) | 87 (14%) | 55 (29%) | <0.001 |
| Antiplatelet therapy (%) | 126 (16%) | 83 (13%) | 43 (23%) | <0.001 |
| Coumarin derivatives (%) | 110 (14%) | 72 (12%) | 38 (20%) | <0.001 |
| Statins (%) | 150 (19%) | 107 (17%) | 43 (23%) | 0.003 |
| Antiarrhythmics (%) | 33 (4%) | 18 (3%) | 15 (8%) | <0.001 |
| Oral antidiabetic therapy (%) | 57 (7%) | 37 (6%) | 20 (11%) | 0.003 |
| Insulin (%) | 35 (4%) | 30 (5%) | 5 (3%) | 0.390 |
Mean ± standard deviation.
ACE, angiotensin-converting enzyme; AT, angiotensin; CABG, coronary artery bypass grafting; CPR, cardiopulmonary resuscitation; CVD, cardiovascular disease; ED, emergency department; PCI, percutaneous coronary intervention; PEA, pulseless electrical activity; ROSC, return of spontaneous circulation; STEMI, ST-segment elevation myocardial infarction; VF, ventricular fibrillation; VT, ventricular tachycardia.
In-hospital characteristics of patients who achieved return of spontaneous circulation
Table 2 summarizes the clinical characteristics, angiography data and outcome of the patients who achieved ROSC. After ROSC, the initial Glasgow Coma Scores were equally low in the elderly and younger cohort, with median scores of 3 (3–5) and 3 (3–6), p = 0.358, respectively. Elderly patients underwent fewer primary CAGs, were less often diagnosed with a STEMI, had smaller creatine kinase-myocardial band (CK-MB) and troponin T levels and presented with higher N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels (p < 0.001 for all). There were no differences in glucose, lactate, pH, and base excess between these groups. Among the elderly, CAG was associated with an initial rhythm of VF, an ST-segment elevation on the ECG and higher arterial pH levels at admission (Supplementary Table 1).
Table 2.
Clinical characteristics, treatment and outcome after return of spontaneous circulation.
| Total n = 551 (68%) |
< 75 years n = 426 (68%) |
⩾ 75 years n = 125 (66%) |
p value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Glasgow Coma Score at admission* | 4 (3, 13) | 4 (3, 14) | 3 (3, 9) | 0.450 |
| STEMI (%) | 183 (33%) | 157 (39%) | 26 (22%) | <0.001 |
| NSTEMI (%) | 72 (13%) | 51 (12%) | 21 (17%) | 0.160 |
| Wall motion abnormalities (%) | 82 (15%) | 73 (32%) | 9 (21%) | 0.150 |
| LVEF (%) | 0.830 | |||
| Poor–moderate | 62 (24%) | 50 (31%) | 12 (33%) | |
| Reasonable–normal | 133 (65%) | 109 (69%) | 24 (67%) | |
| Laboratory values | ||||
| Glucose* | 12.7 (9.9, 16.9) | 12.3 (9.6, 16.6) | 13.2 (10.6, 17.1) | 0.060 |
| Lactate* | 6.2 (2.95, 9.75) | 6.3 (2.9, 9.9) | 5.4 (3.1, 8.7) | 0.470 |
| pH* | 7.25 (7.10, 7.33) | 7.25 (7.1, 7.32) | 7.27 (7.11, 7.34) | 0.510 |
| Base excess* | −8.1 (−14.5, −3.9) | −8.1 (−14.7, −3.8) | −7.9 (−12.9, −3.95) | 0.640 |
| CK max* | 1943 (436, 5585) | 2390 (607, 6376) | 628 (286, 3104) | <0.001 |
| CK-MB max* | 169 (64, 554) | 214 (77, 587) | 110 (44, 241) | <0.001 |
| Troponin T max* | 2.22 (0.29, 11.33) | 2.53 (0.39, 12.30) | 0.99 (0.20, 6.30) | 0.016 |
| NT-pro BNP* | 313 (75, 1607) | 229 (69, 898) | 1731 (312, 3619) | <0.001 |
| Treatment | ||||
| Coronary angiography (%) | 295 (54%) | 255 (60%) | 40 (32%) | <0.001 |
| Primary | 217 (39%) | 185 (43%) | 32 (26%) | <0.001 |
| Secondary | 78 (14%) | 70 (16%) | 8 (6%) | 0.005 |
| Door to balloon time (min)* | 88 (56, 128) | 88 (55, 125) | 83 (61, 137) | 0.270 |
| Culprit lesion known (%) | 295 (54%) | 255 (60%) | 40 (32%) | 0.089 |
| Right coronary artery | 67 (12%) | 58 (14%) | 9 (7%) | |
| Left anterior descending | 111 (20%) | 97 (23%) | 14 (11%) | |
| Circumflex | 44 (8%) | 37 (9%) | 7 (6%) | |
| Left main | 12 (2%) | 7 (2%) | 5 (4%) | |
| Graft | 4 (1%) | 4 (1%) | 0 (0%) | |
| Unknown or multiple | 32 (6%) | 28 (7%) | 4 (3%) | |
| No culprit | 25 (5%) | 24 (6%) | 1 (1%) | |
| PCI (%) | 215 (39%) | 184 (43%) | 31 (25%) | <0.001 |
| Primary | 191 (35%) | 164 (39%) | 27 (22%) | <0.001 |
| Secondary | 24 (4%) | 20 (5%) | 4 (2%) | 0.470 |
| Intra-aortic balloon pump (%) | 73 (13%) | 60 (14%) | 13 (10%) | 0.290 |
| Mild therapeutic hypothermia (%) | 201 (37%) | 161 (38%) | 40 (32%) | 0.280 |
| Mechanical ventilation (%) | 145 (26%) | 118 (28%) | 27 (22%) | 0.200 |
| Outcome | ||||
| Length of hospital stay (days)* | 6 (3, 18) | 7 (3, 20) | 4 (2,12.5) | <0.001 |
| In-hospital death (%) | 268 (49%) | 184 (43%) | 84 (67%) | <0.001 |
| Neurological bad outcome | 184 (33%) | 132 (31%) | 52 (42%) | |
| Cardiogenic shock | 43 (8%) | 25 (6%) | 18 (14%) | |
| Multiorgan failure | 17 (3%) | 12 (3%) | 5 (4%) | |
| Other | 24 (4%) | 15 (4%) | 9 (7%) | |
| Hospital survival (%) | 283 (51%) | 242 (57%) | 41 (33%) | <0.001 |
| CPC at discharge (%) | 0.041 | |||
| 1 | 239 (84%) | 209 (86%) | 30 (71%) | |
| 2 | 36 (13%) | 27 (11%) | 9 (21%) | |
| 3 | 9 (3%) | 6 (3%) | 3 (7%) | |
| Survival at end of follow up (%) | 185 (33%) | 178 (42%) | 7 (6%) | <0.001 |
Median (interquartile range).
CK-MB, creatine kinase-myocardial band; CPC, cerebral performance category; LVEF, left ventricular ejection fraction; (N)STEMI, (non)-ST-segment elevation myocardial infarction; NT-pro BNP, N-terminal prohormone of brain natriuretic peptide; PCI, percutaneous coronary intervention; ROSC, return of spontaneous circulation.
Treatment
Only 31 patients (25%) from the elderly group underwent PCI versus 184 patients (43%) in the younger group (p < 0.001). In both younger and elderly patients, independent predictors of PCI were the presence of ST-segment elevation on the ECG and higher serum CK-MB levels [Supplementary Table 2(a), 2(b)]. MTH was similarly used in the elderly (32%, 95% CI 24–41%) and younger (38%, 95% CI 33–43%) patients (p = 0.277). In the elderly, an initial rhythm of VF and higher serum CK-MB levels were independently associated with institution of MTH (Supplementary Table 3). There were no statistically significant differences in the use of an intra-aortic balloon pump and mechanical ventilation between both groups (Table 2).
Outcome
Survival to hospital discharge was lower in elderly patients with only 33% (95% CI 25–42%) surviving compared with 57% (95% CI 52–62%) in the younger group (p < 0.001; Table 2). Being elderly was independently associated with in-hospital mortality when adjusted for known prognostic factors [i.e. sex, initial rhythm, bystander CPR, witnessed arrest, arterial glucose and pH levels, and prior CVD (Supplementary Table 4)]. Of the 41 elderly patients who survived to hospital discharge, 24 (59%, 95% CI 42–74%) had VF versus 17 (41%, 95% CI 26–58%) patients with non-VF (p = 0.600). In younger patients, survival in the VF group was 207 (86%, 95% CI 81–90%) versus 35 (15%, 95% CI 10–20%) in the non-VF patients (p < 0.001). Only Glasgow Coma Score at admission was independently associated with mortality in the elderly (Supplementary Table 5). Length of hospital stay was shorter in the elderly patients (Table 2), but length of hospital stay was similar for hospital survivors in both groups.
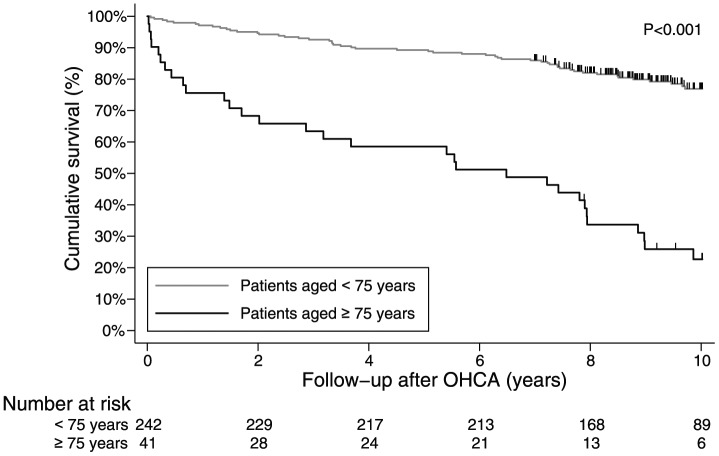
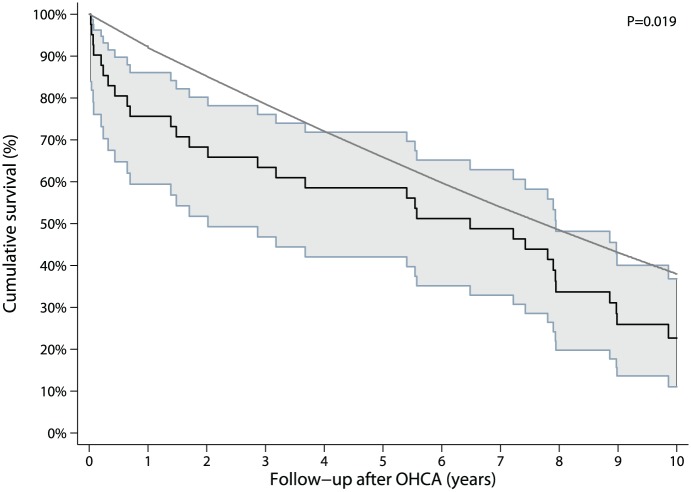
After hospital discharge, survival in elderly patients was worse in comparison with younger patients (Figure 2). The 1-year survival in the overall OHCA group after hospital discharge was 94% (95% CI 91–97%) and 5-year survival was still 85% (95% CI 80–89%). In contrast, in the patients aged ⩾ 75 years, 1-year survival was only 76% (95% CI 60–88%) and 5-year survival dropped to 59% (95% CI 42–74%). However, when standardized for sex, age and calendar year, long-term survival approached that of the general population (Figure 3). The median survival of the elderly patients was 6.5 (95% CI 2.0–7.9) years compared with 7.7 (95% CI 7.5–7.9) years for a matched general population (p = 0.019).
Figure 2.
Survival curves of younger (< 75 years) and elderly (⩾ 75 years) patients.
OHCA, out-of-hospital cardiac arrest.
Figure 3.
Survival curve elderly versus matched control population with 95% confidence intervals.
Neurological outcome at hospital discharge
The younger cohort had a better neurological outcome, as scored by the CPC (Table 2). In the elderly, 73% (95% CI 57–86%) had a CPC of 1 compared with 86% (95% CI 81–90%) in the younger patients (p = 0.031). The incidence of poor outcome (CPC > 2) was 7.3% (95% CI 1.5–20%) in the elderly versus 2.5% (95% CI 0.9–5.3%) in the younger cohort (p = 0.103).
Cause of death
Causes of in-hospital death did not vary substantially between age groups (Table 2). The major cause of death was neurological damage that was deemed not compatible with survival. Other important causes of death were secondary circulatory failure and multiorgan failure (Table 2). Of the 35 patients with a known malignancy, only one survived until end of follow up (p = 0.003).
Discussion
From the long-term follow up of a cohort of 810 patients after OHCA, two main observations emerged. First, despite similar percentages of patients regaining spontaneous circulation, in-hospital mortality was considerably higher in elderly compared with younger patients. Second, we add to the current literature, that elderly patients who were discharged alive had a median survival of 6.5 years compared with 7.7 years in matched controls in the general population. Moreover, in these elderly patients, the neurological outcome was good in a high percentage of patients, with less than 10% with a poor neurological outcome.
Our findings confirm that elderly patients have worse outcomes than younger patients: Funada and colleagues studied 334,730 elderly (⩾ 75 years) and 23,520 very elderly (⩾ 95 years) patients,12,33 and in 2017, five more studies described worse outcomes of elderly patients after 1 month: Aissaoui and colleagues (n = 464, ⩾ 65 years),34 Andrew and colleagues (n = 14,678, ⩾ 65 years),18 Segal and colleagues (n = 18,249, > 65 years),14 Sulzgruber and colleagues (n = 1315, ⩾ 65 years),15 and Wiel and colleagues (n = 4347, > 65 years).13 In a study with 34,291 patients Wong and colleagues showed in 2014 that over time, the survival of the elderly at 1 year has increased.36 The favorable outcome trend for functional outcome reflected by good CPC scores at 1 month was recently shown in large Japanese cohort studies.12,37,38 Beesems and colleagues showed worse survival and equally favorable functional outcome in survivors compared with our study.39 In 1332 patients aged ⩾ 70 years, 1-year survival in patients discharged alive was 88% and neurologic outcome, as defined by a CPC of 1 or 2, was good in 90% of patients. Winther-Jensen and colleagues showed that 70% of the very elderly patients (i.e. ⩾ 80 years) had CPC scores of 1 or 2 at 30-day follow up, which is comparable with the observed 70% elderly with a CPC score of 1 in our cohort.17 Studies are unanimous on the worse short-term outcome of elderly after OHCA, but we observe that healthy aging among the survivors seems possible.
In our study, several factors were associated with both mortality and unfavorable neurological outcome. In line with observations of others, the elderly have a higher cardiovascular risk profile and more comorbidities.39–41 We also observed a higher incidence of an initial rhythm other than VF, which is associated with a poor outcome, in the elderly patients.42 Similar to Sulzgruber and colleagues, the initial rhythm and other known prognostic factors such as witnessed arrest, bystander CPR, arterial glucose, base excess and lactate levels were not associated with outcome in the elderly.15 More data are needed to identify early prognostic variables, as other cohort studies did find associations between these variables and outcome.6,42–44
Compared with other studies, the incidence of ROSC in the elderly in our population was high and almost equal to younger patients.47,48 A selection bias could be present as referral to our hospital may have been considered futile in patients with very poor early prognostic variables or a nonshockable rhythm present for more than 20 minutes. Like in the study of Winther-Jensen and colleagues, CAG and PCI were employed less frequently in the elderly patients.17 Our data show that in elderly patients, OHCA less frequently has an acute cardiac origin. Elderly patients less often suffer from a myocardial infarction, initial rhythm of VF and have lower cardiac biomarkers. Also, the higher incidence of previous myocardial infarction in the elderly might underlie secondary rhythm disturbances that cause OHCA. The different underlying pathology makes them less suitable for treatment such as PCI.6,49,50 Another reason could be that elderly patients tend to receive a less aggressive diagnostic work up compared with younger patients.13,51–53 MTH was employed equally in both groups in our cohort.
Treatment decisions can be particularly difficult for clinicians caring for elderly patients. CPR for older patients is a controversial issue.16,54,55 Initiating CPR in the elderly is a decision that is affected by sometimes conflicting opinions for what is best for the patient, and these opinions may vary from country to country. It involves not only medical, but also ethical decisions, especially when there are important associated comorbidities that directly affect the patient’s prognosis.21 As shown in our study, the incidence of malignancies is significantly higher in the elderly. Since long-term survival of elderly hospital survivors is reasonable with a median survival of 6.5 years, our data thus do not support the withholding of resuscitation based on age only.16
Limitations
This study has several limitations. First, it was performed retrospectively on data of patients admitted to our hospital. Detailed data are lacking on the person who witnessed the arrest (EMS or bystander) and time frames of initial treatment, such as time to CPR, ACLS and ROSC for instance. Second, not all treatment was standardized; various potential sources of unidentified bias such as confounding by indication in the care of older and younger patients may be present. However, highlighting this potential bias is also an important finding in this study. Finally, these results only relate to patients presenting to the emergency unit of a single center. This limits the external validity of our findings. Some patients who were resuscitated during the study period in our region may not have been referred to our hospital, since PCI was not deemed necessary for some patients. This may explain differences in our patient population in comparison with other studies.10,56 As such, the outcome data we present do not pertain to the total population of patients suffering OHCA in our region.
Conclusion
Despite similar rates of ROSC after OHCA in elderly and younger patients, hospital survival for elderly patients was approximately half that of younger patients. However, the majority of elderly patients who survived initial treatment had a CPC score of 1 and a lifespan that approximates that of the general population.
Supplemental Material
Supplemental material, Supplements for Long-term outcome of elderly out-of-hospital cardiac arrest survivors as compared with their younger counterparts and the general population by Bart Hiemstra, Remco Bergman, Anthony R. Absalom, Joukje van der Naalt, Pim van der Harst, Ronald de Vos, Wybe Nieuwland, Maarten W. Nijsten and Iwan C. C. van der Horst in Therapeutic Advances in Cardiovascular Disease
Acknowledgments
Bart Hiemstra and Remco Bergman contributed equally to this work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Bart Hiemstra  https://orcid.org/0000-0001-6547-2138
https://orcid.org/0000-0001-6547-2138
Anthony R Absalom  https://orcid.org/0000-0001-7563-9157
https://orcid.org/0000-0001-7563-9157
Contributor Information
Bart Hiemstra, Department of Critical Care, University of Groningen, University Medical Center Groningen, Hanzeplein 1, PO Box 30.001, Groningen, 9700 RB, The Netherlands.
Remco Bergman, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Anthony R. Absalom, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
Joukje van der Naalt, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Pim van der Harst, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Ronald de Vos, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Wybe Nieuwland, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Maarten W. Nijsten, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
Iwan C. C. van der Horst, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
References
- 1. Nolan JP. Optimizing outcome after cardiac arrest. Curr Opin Crit Care 2011; 17: 520–526. [DOI] [PubMed] [Google Scholar]
- 2. Savastano S, Klersy C, Raimondi M, et al. Positive trend in survival to hospital discharge after out-of-hospital cardiac arrest: a quantitative review of the literature. J Cardiovasc Med (Hagerstown) 2014; 15: 609–615. [DOI] [PubMed] [Google Scholar]
- 3. Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2016; 2: CD004128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bro-Jeppesen J, Kjaergaard J, Horsted TI, et al. The impact of therapeutic hypothermia on neurological function and quality of life after cardiac arrest. Resuscitation 2009; 80: 171–176. [DOI] [PubMed] [Google Scholar]
- 5. Kim WY, Giberson TA, Uber A, et al. Neurologic outcome in comatose patients resuscitated from out-of-hospital cardiac arrest with prolonged downtime and treated with therapeutic hypothermia. Resuscitation 2014; 85: 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergman R, Hiemstra B, Nieuwland W, et al. Long-term outcome of patients after out-of-hospital cardiac arrest in relation to treatment: a single-centre study. Eur Heart J Acute Cardiovasc Care 2016; 5: 328–338. [DOI] [PubMed] [Google Scholar]
- 7. Hosmane VR, Mustafa NG, Reddy VK, et al. Survival and neurologic recovery in patients with ST-segment elevation myocardial infarction resuscitated from cardiac arrest. J Am Coll Cardiol 2009; 53: 409–415. [DOI] [PubMed] [Google Scholar]
- 8. Lang ES. ACP Journal Club. Review: therapeutic hypothermia improves neurologic outcome and survival to discharge after cardiac arrest. Ann Intern Med 2010; 152: 22. [DOI] [PubMed] [Google Scholar]
- 9. Hinchey PR, Myers JB, Lewis R, et al. Improved out-of-hospital cardiac arrest survival after the sequential implementation of 2005 AHA guidelines for compressions, ventilations, and induced hypothermia: the Wake County experience. Ann Emerg Med 2010; 56: 348–357. [DOI] [PubMed] [Google Scholar]
- 10. Berdowski J, Berg RA, Tijssen JG, et al. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation 2010; 81: 1479–1487. [DOI] [PubMed] [Google Scholar]
- 11. Van der Wal G, Brinkman S, Bisschops LL, et al. Influence of mild therapeutic hypothermia after cardiac arrest on hospital mortality. Crit Care Med 2011; 39: 84–88. [DOI] [PubMed] [Google Scholar]
- 12. Funada A, Goto Y, Maeda T, et al. Improved survival with favorable neurological outcome in elderly individuals with out-of-hospital cardiac arrest in Japan – a nationwide observational cohort study. Circ J 2016; 80(5): 1153–1162. [DOI] [PubMed] [Google Scholar]
- 13. Wiel E, Di Pompeo C, Segal N, et al. Age discrimination in out-of-hospital cardiac arrest care: a case-control study. Eur J Cardiovasc Nurs. Epub ahead of print 1 December 2017. DOI: 10.1177/1474515117746329. [DOI] [PubMed] [Google Scholar]
- 14. Segal N, di Pompeo C, Escutnaire J, et al. Evolution of survival in cardiac arrest with age in elderly patients: is resuscitation a dead end? J Emerg Med 2017; 54(3): 295–301. [DOI] [PubMed] [Google Scholar]
- 15. Sulzgruber P, Sterz F, Poppe M, et al. Age-specific prognostication after out-of-hospital cardiac arrest - the ethical dilemma between ‘life-sustaining treatment’ and ‘the right to die’ in the elderly. Eur Heart J Acute Cardiovasc Care 2017; 6(2): 112–120. [DOI] [PubMed] [Google Scholar]
- 16. Van de Glind EM, van Munster BC, van de Wetering FT, et al. Pre-arrest predictors of survival after resuscitation from out-of-hospital cardiac arrest in the elderly a systematic review. BMC Geriatr 2013; 13: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winther-Jensen M, Pellis T, Kuiper M, et al. Mortality and neurological outcome in the elderly after target temperature management for out-of-hospital cardiac arrest. Resuscitation 2015; 91: 92–98. [DOI] [PubMed] [Google Scholar]
- 18. Andrew E, Mercier E, Nehme Z, et al. Long-term functional recovery and health-related quality of life of elderly out-of-hospital cardiac arrest survivors. Resuscitation 2018; 126: 118–124. [DOI] [PubMed] [Google Scholar]
- 19. Applebaum GE, King JE, Finucane TE. The outcome of CPR initiated in nursing homes. J Am Geriatr Soc 1990; 38: 197–200. [DOI] [PubMed] [Google Scholar]
- 20. Nagappan R, Parkin G. Geriatric critical care. Crit Care Clin 2003; 19: 253–270. [DOI] [PubMed] [Google Scholar]
- 21. Edin MG. Cardiopulmonary resuscitation in the frail elderly: clinical, ethical and halakhic issues. Isr Med Assoc J 2007; 9: 177–179. [PubMed] [Google Scholar]
- 22. Andrew E, Nehme Z, Wolfe R, et al. Long-term survival following out-of-hospital cardiac arrest. Heart 2017; 103(14): 1104–1110. [DOI] [PubMed] [Google Scholar]
- 23. Smith K, Andrew E, Lijovic M, et al. Quality of life and functional outcomes 12 months after out-of-hospital cardiac arrest. Circulation 2015; 131(2): 174–181. [DOI] [PubMed] [Google Scholar]
- 24. Kragholm K, Wissenberg M, Mortensen RN, et al. Bystander efforts and 1-year outcomes in out-of-hospital cardiac arrest. N Engl J Med 2017; 376: 1737–1747. [DOI] [PubMed] [Google Scholar]
- 25. Nichol G, Guffey D, Stiell IG, et al. Post-discharge outcomes after resuscitation from out-of-hospital cardiac arrest: a ROC PRIMED substudy. Resuscitation 2015; 93: 74–81. [DOI] [PubMed] [Google Scholar]
- 26. Mahmoud KD, Gu YL, Nijsten MW, et al. Interhospital transfer due to failed prehospital diagnosis for primary percutaneous coronary intervention: an observational study on incidence, predictors, and clinical impact. Eur Heart J Acute Cardiovasc Care 2013; 2: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soar J, Nolan JP, Bottiger BW, et al. European Resuscitation Council guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation 2015; 95: 100–147. [DOI] [PubMed] [Google Scholar]
- 28. Peels HO, Jessurun GA, Van der Horst IC, et al. Outcome in transferred and nontransferred patients after primary percutaneous coronary intervention for ischaemic out-of-hospital cardiac arrest. Catheter Cardiovasc Interv 2008; 71(2): 147–151. [DOI] [PubMed] [Google Scholar]
- 29. Wijdicks EF, Hijdra A, Young GB, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006; 67: 203–210. [DOI] [PubMed] [Google Scholar]
- 30. Bouwes A, Binnekade JM, Kuiper MA, et al. Prognosis of coma after therapeutic hypothermia: a prospective cohort study. Ann Neurol 2012; 71: 206–212. [DOI] [PubMed] [Google Scholar]
- 31. Central Bureau for Statistics. Statistics Netherlands: life expectancy; gender and age from 1950 on (per year), http://statline.cbs.nl/StatWeb/publication/?PA=37360ned (2015, accessed 13 September 2017).
- 32. Jakobsen JC, Gluud C, Wetterslev J, et al. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol 2017; 17(1): 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Funada A, Goto Y, Maeda T, et al. Prehospital predictors of neurological outcomes in out-of-hospital cardiac arrest patients aged 95 years and older: a nationwide population-based observational study. J Cardiol 2017; 69(1): 340–344. [DOI] [PubMed] [Google Scholar]
- 34. Aissaoui N, Bougouin W, Dumas F, et al. Post-resuscitation care in elderly patients: insights from the sudden death expertise center registry. Eur Heart J 2017; 38(1): ehx504.P3022. [DOI] [PubMed] [Google Scholar]
- 35. Wong MK, Morrison LJ, Qiu F, et al. Trends in short-and long-term survival among out-of-hospital cardiac arrest patients alive at hospital arrival. Circulation 2014; 130: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 36. SOS-KANTO 2012 Study Group. Changes in treatments and outcomes among elderly patients with out-of-hospital cardiac arrest between 2002 and 2012: a post hoc analysis of the SOS-KANTO 2002 and 2012. Resuscitation 2015; 97: 76–82. [DOI] [PubMed] [Google Scholar]
- 37. Kitamura T, Iwami T, Kawamura T, et al. Nationwide improvements in survival from out-of-hospital cardiac arrest in Japan. Circulation 2012; 126(24): 2834–2843. [DOI] [PubMed] [Google Scholar]
- 38. Beesems SG, Blom MT, Van der Pas MH, et al. Comorbidity and favorable neurologic outcome after out-of-hospital cardiac arrest in patients of 70 years and older. Resuscitation 2015; 94: 33–39. [DOI] [PubMed] [Google Scholar]
- 39. Bagnall AJ, Goodman SG, Fox KA, et al. Influence of age on use of cardiac catheterization and associated outcomes in patients with non-ST-elevation acute coronary syndromes. Am J Cardiol 2009; 103: 1530–1536. [DOI] [PubMed] [Google Scholar]
- 40. Mosier J, Itty A, Sanders A, et al. Cardiocerebral resuscitation is associated with improved survival and neurologic outcome from out-of-hospital cardiac arrest in elders. Acad Emerg Med 2010; 17: 269–275. [DOI] [PubMed] [Google Scholar]
- 41. Wissenberg M, Lippert FK, Folke F, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA 2013; 310: 1377–1384. [DOI] [PubMed] [Google Scholar]
- 42. McGill JW, Ruiz E. Central venous pH as a predictor of arterial pH in prolonged cardiac arrest. Ann Emerg Med 1984; 13: 684–687. [DOI] [PubMed] [Google Scholar]
- 43. Mullner M, Sterz F, Domanovits H, et al. The association between blood lactate concentration on admission, duration of cardiac arrest, and functional neurological recovery in patients resuscitated from ventricular fibrillation. Intensive Care Med 1997; 23: 1138–1143. [DOI] [PubMed] [Google Scholar]
- 44. Takasu A, Sakamoto T, Okada Y. Arterial base excess after CPR: the relationship to CPR duration and the characteristics related to outcome. Resuscitation 2007; 73: 394–399. [DOI] [PubMed] [Google Scholar]
- 45. Fukuda T, Ohashi-Fukuda N, Matsubara T, et al. Trends in outcomes for out-of-hospital cardiac arrest by age in Japan: an observational study. Medicine (Baltimore) 2015; 94: e2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andersen LW, Bivens MJ, Giberson T, et al. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation 2015; 94: 49–54. [DOI] [PubMed] [Google Scholar]
- 47. Hollenbeck RD, McPherson JA, Mooney MR, et al. Early cardiac catheterization is associated with improved survival in comatose survivors of cardiac arrest without STEMI. Resuscitation 2014; 85: 88–95. [DOI] [PubMed] [Google Scholar]
- 48. Camuglia AC, Randhawa VK, Lavi S, et al. Cardiac catheterization is associated with superior outcomes for survivors of out of hospital cardiac arrest: review and meta-analysis. Resuscitation 2014; 85: 1533–1540. [DOI] [PubMed] [Google Scholar]
- 49. Scott IA, Guyatt GH. Cautionary tales in the interpretation of clinical studies involving older persons. Arch Intern Med 2010; 170(7): 587–595. [DOI] [PubMed] [Google Scholar]
- 50. Lindner TW, Langorgen J, Sunde K, et al. Factors predicting the use of therapeutic hypothermia and survival in unconscious out-of-hospital cardiac arrest patients admitted to the ICU. Crit Care 2013; 17(4): R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hirlekar G, Karlsson T, Aune S, et al. Survival and neurological outcome in the elderly after in-hospital cardiac arrest. Resuscitation 2017; 118: 101–106. [DOI] [PubMed] [Google Scholar]
- 52. Gordon M. Ethical challenges in end-of-life therapies in the elderly. Drugs Aging 2002; 19: 321–329. [DOI] [PubMed] [Google Scholar]
- 53. Deasy C, Bray JE, Smith K, et al. Out-of-hospital cardiac arrests in the older age groups in Melbourne, Australia. Resuscitation 2011; 82: 398–403. [DOI] [PubMed] [Google Scholar]
- 54. Van Alem AP, Chapman FW, Lank P, et al. A prospective, randomised and blinded comparison of first shock success of monophasic and biphasic waveforms in out-of-hospital cardiac arrest. Resuscitation 2003; 58: 17–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplements for Long-term outcome of elderly out-of-hospital cardiac arrest survivors as compared with their younger counterparts and the general population by Bart Hiemstra, Remco Bergman, Anthony R. Absalom, Joukje van der Naalt, Pim van der Harst, Ronald de Vos, Wybe Nieuwland, Maarten W. Nijsten and Iwan C. C. van der Horst in Therapeutic Advances in Cardiovascular Disease