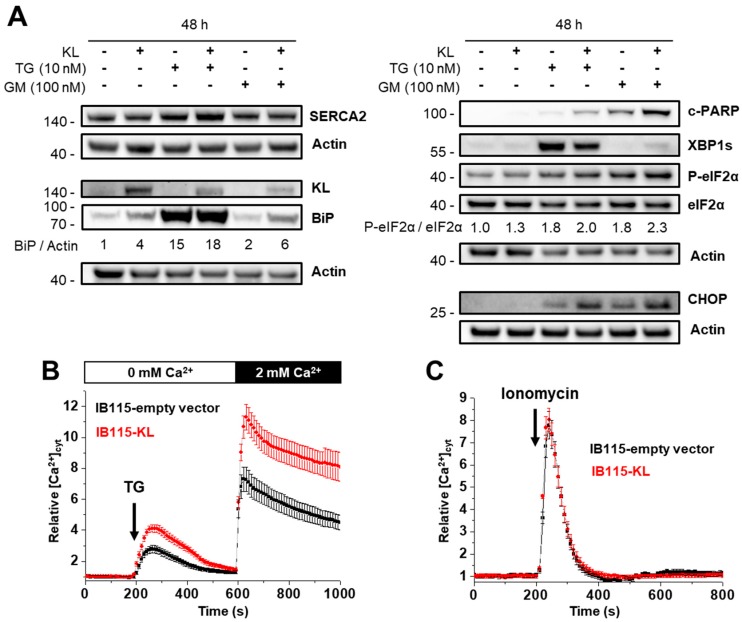
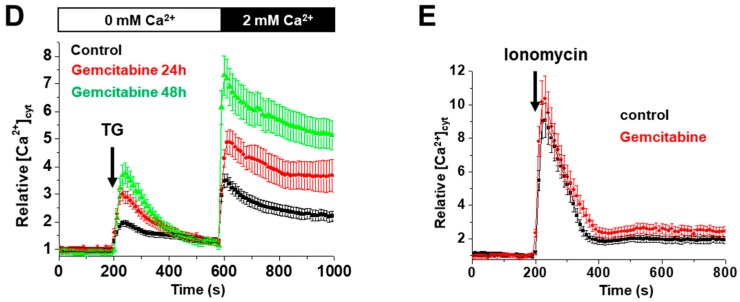
Figure 4.
KL-overexpression sensitizes DDLPS to endoplasmic reticulum (ER) stress by disrupting the intracellular Ca2+ homeostasis. (A) Abundance and phosphorylation ratios of proteins implicated in ER stress were analyzed by western blotting after 48 h of incubation with no drugs, 10 nM thapsigargin (TG) or 100 nM gemcitabine. Actin was used as a loading control. To compare the ratios between phosphorylated form and the total protein during treatments, the results were all normalized to control condition (IB115-empty vector, no treatment) set to 1. Results are representative of three independent experiments. (B–E) Variations of relative cytosolic Ca2+ concentration were monitored by fluorescence videomicroscopy in Fluo2-loaded cells bathed in a Ca2+-free HBSS medium. Results are represented as means ± SD. (B,D) TG (10 nM) was added at 200 s to evaluate the intracellular Ca2+ mobilization and (B) 2 mM Ca2+ was applied at 600 s to measure the capacitive Ca2+-entry. (C,E) Ionomycin (100 nM) was added at 200 s to visualize the ER Ca2+-content. (B) KL overexpression increased intracellular Ca2+ response to TG and the subsequent capacitive Ca2+ entry (n = 143 and n = 183 for the IB115-empty vector and IB115-cells, respectively), but (C) did not affect the Ca2+ ER content, compared to IB115-empty vector cell line (n = 79 and n = 123 for the IB115-empty vector and IB115-cells, respectively). (D,E) IB115-empty vector cells were pretreated with gemcitabine (100 nM) during 24 and 48 h. Gemcitabine increased (D) TG-induced Ca2+ response (control n = 96; 24 h, n = 82; 48 h, n = 34) but (E) did not alter the size of the reticular Ca2+ pools (control n = 102; gemcitabine n = 81).