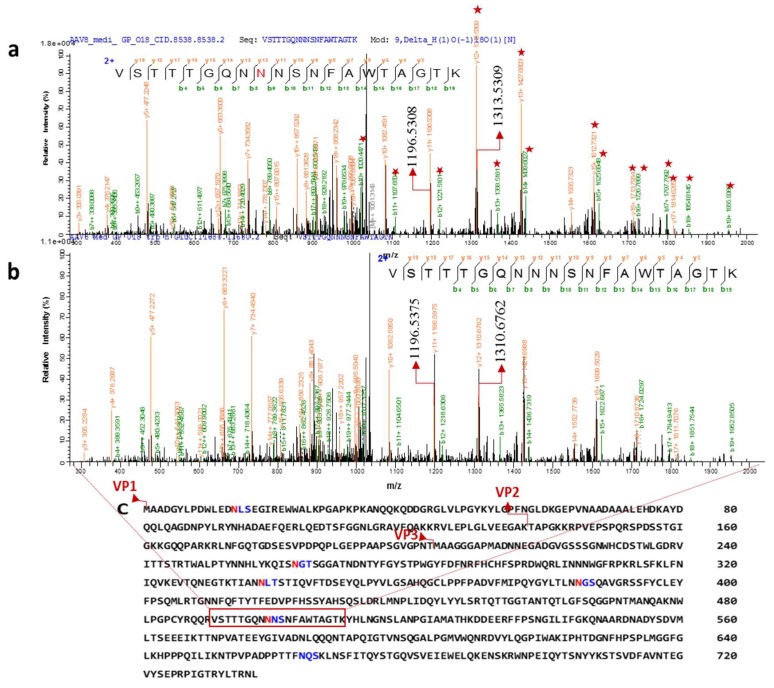
Figure 4.
Two distinct tandem mass spectra of peptide sequence “VSTTTGQNNNSNFAWTAGTK” of the AAV8 capsid protein (in the common region of VPs). Characterized CID spectra of glycosylated peptide after 18O mediated digestion. (a) The position of de-N-glycosylated asparagine 499 (18O-incorporated aspartic acid) was attested by mass increment of 2.8547 Da (theoretical mass difference 2.98 Da) to the series of b and y ion (the precursor ion mass: m/z 2101.93193). (b) The position of Non-glycosylated asparagine 499 in the sequence was confirmed by y12/b8 ions (the precursor ion mass: m/z 2099.0766 Da). The highlighted ions (marked with a star) shows the mass differences from the non-glycosylated peptide mass spectrum. y11+ (highlighted) ions are same in both the spectra. (c) Capsid protein amino acid sequence and possible N-glycosylation sites predicted by NetNglyc software based on the consensus sequence (NXT/S, “X” can be any amino acid except proline). Different N-terminal sequence of VP1, VP2 and VP3 are marked in the sequence. The glycosite identified peptide is highlighted on the sequence.