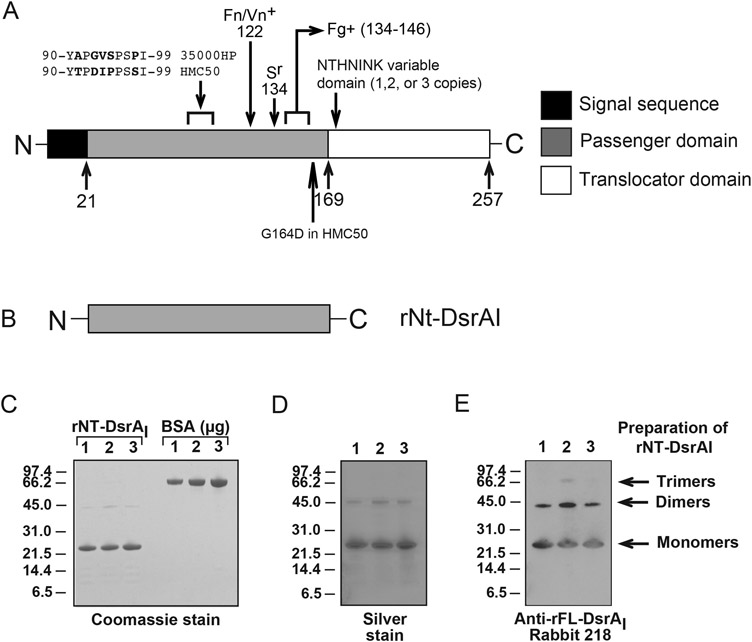
Figure 1. rNT-DsrAI as a vaccine immunogen against experimental chancroid.
A. Schematic representation of the DsrA protein showings its different domains, the sections involved in its different functions and the amino acids that vary between challenge strains. The arrows and numbers at the bottom of the cartoon indicate the amino acid at the end of each domain. Removal of the passenger domain of DsrA abrogates Fg, Vn and Fg binding and renders H. ducreyi serum sensitive in the presence of 50% normal human serum [7, 15]. These functions are regained (Fg+, Fn/Vn+, Sr) when the indicated C-terminal portions of the passenger domain of DsrA are expressed along with the translocator domain in an isogenic dsrA mutant strain [7, 15]. B. The passenger domain of rDsrAI, termed rNT-DsrAI, was used as the immunogen. C. Coomassie stained gel of the three preparations of rNT-DsrAI used in vaccination studies. Bovine serum albumin (BSA) was used to confirm concentration of the purified protein, in addition to determination of protein concentration using a commercially available kit. D. Silver stain of the same preparations in C. E. Western blot analysis of rNT-DsrAI preparations with a polyclonal Ab against full-length DsrA (rFL-DsrAI) [16]. Sr, serum resistance; Fn, fibronectin; Fg, fibrinogen; Vn, vitronectin; + indicates region of DsrA involved in binding to the particular protein.