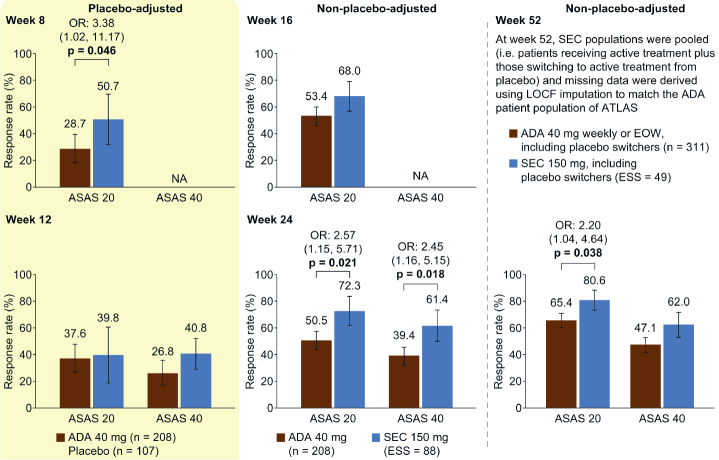
Figure 4.
MEASURE 2 MAIC analysis. ASAS 20 and ASAS 40 response rates placebo-adjusted at weeks 8 and 12 and non-placebo-adjusted at weeks 16, 24, and 52.
All p values (shown when significant, p<0.05) were derived from OR values. Error bars and figures in brackets show 95% confidence intervals. Numbers above each bar are the absolute mean response rate (ATLAS) and the mean response rate after reweighting (MEASURE 2)
ADA: adalimumab, ASAS 20/40: 20%/40% improvement in the Assessment of SpondyloArthritis International Society response criteria, EOW: every other week, ESS: effective sample size, LOCF: last observation carried forward, MAIC: matching-adjusted indirect comparison, NA: not available, OR: odds ratio, SEC: secukinumab