Figure 1.
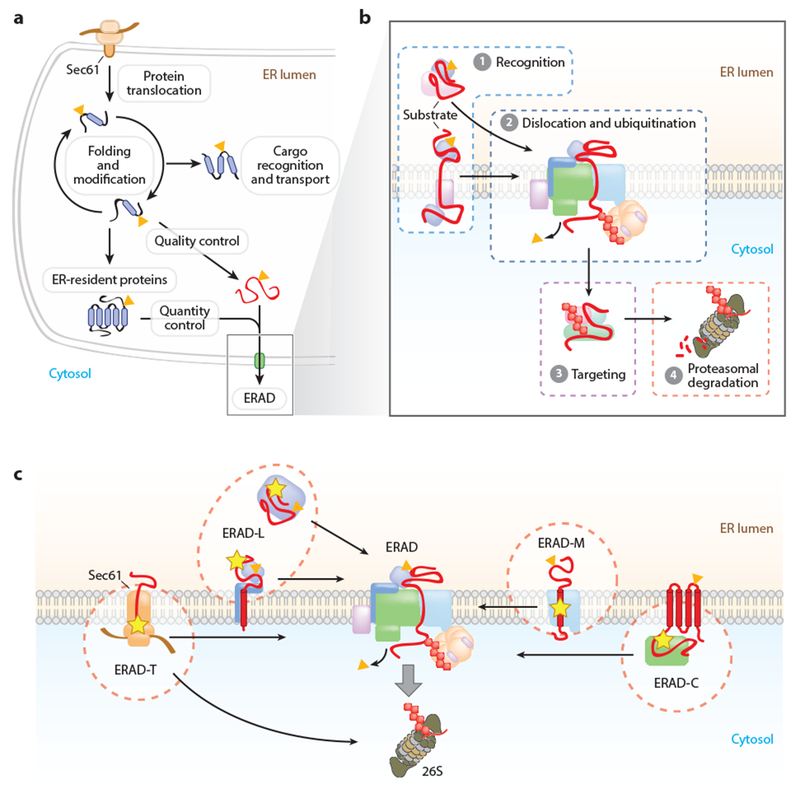
Mechanisms of protein quality and quantity control in the endoplasmic reticulum (ER). (a) Protein folding and maturation in the ER. Proteins inserted through the Sec61 translocon are subject to cotranslational and posttranslational folding. Proteins that have achieved their native conformation are recognized and transported to their final cellular destination. Proteins failing to achieve their proper conformation are triaged for ER-associated degradation (ERAD) (quality control). The levels of some properly folded proteins are also controlled by regulated degradation by ERAD (quantity control). (b) ERAD mediates the delivery of proteins from the ER to the cytosolic 26S proteasome through a series of coupled steps: ① substrate recognition, ② dislocation and ubiquitin conjugation, and ③ targeting of the ubiquitinated proteins to the proteasome for ④ proteolysis. (c) The ERAD system comprises multiple pathways that mediate the recognition and degradation of topologically diverse substrates. These pathways are commonly designated based upon the location of the substrate degradation signal: luminal (ERAD-L), cytosolic (ERAD-C), membrane (ERAD-M), or translocon (ERAD-T). (Yellow star indicates degradation signal; orange triangle indicates N-glycan.)
