Table 1.
The calculated lipophilicities (log P/Clog P), electronic σ parameters and IC50 [μmol/L] values related to PET inhibition in spinach chloroplasts of quinoline-2-carboxamides 1–19c in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard. NF = not found in literature, ND = not determined due to precipitation during the experiment or interaction with 2,6-dichlorophenol-indophenol (DCIPP).
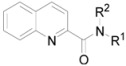 | |||||
| Comp. | R1 | R2 | PET inhibition IC50 [μmol/L] | log P ACD/Log P | σ [35] |
|---|---|---|---|---|---|
| 1 | i-Pr | H | ND | 2.02 ± 0.34 | −0.19 [36] |
| 2 | –C12H25 | H | 573 | 6.98 ± 0.32 | NF |
| 3 | –(CH2)4– | 1598 | 1.15 ± 0.26 | NF | |
| 4 | –(CH2)5– | ND | 1.72 ± 0.26 | NF | |
| 5 | c-Pn | H | 762 | 2.63 ± 0.33 | −0.20 [36] |
| 6 | c-Hx | H | 1041 | 3.20 ± 0.33 | −0.15 [36] |
| 7 | c-Hp | H | ND | 3.76 ± 0.33 | NF |
| 8 | c-Oc | H | 415 | 4.33 ± 0.33 | NF |
| 9 | Ph | H | 85.1 | 2.90 ± 0.34 | 0.60 [36]/0 |
| 10 | Bn | H | 59.4 | 2.91 ± 0.35 | 0.22 [36] |
| 11 | –C2H4Ph | H | ND | 3.33 ± 0.33 | 0.08 [36] |
| 12a | 2-OH-Ph | H | 16.3 | 2.54 ± 0.36 | −0.09 |
| 12b | 3-OH-Ph | H | ND | 2.55 ± 0.36 | 0.12 |
| 12c | 4-OH-Ph | H | ND | 2.15 ± 0.35 | −0.37 |
| 13a | 2-OCH3-Ph | H | ND | 2.80 ± 0.36 | −0.39 [37] |
| 13b | 3-OCH3-Ph | H | ND | 3.06 ± 0.36 | 0.12 |
| 13c | 4-OCH3-Ph | H | ND | 2.85 ± 0.36 | −0.27 |
| 14a | 2-CH3-Ph | H | 142.9 | 3.36 ± 0.34 | NF |
| 14b | 3-CH3-Ph | H | 100.6 | 3.36 ± 0.34 | −0.07 |
| 14c | 4-CH3-Ph | H | ND | 3.36 ± 0.34 | −0.17 |
| 15a | 2-F-Ph | H | 98.1 | 2.86 ± 0.44 | 0.24 [38] |
| 15b | 3-F-Ph | H | 86.9 | 3.38 ± 0.44 | 0.34 |
| 15c | 4-F-Ph | H | 75.3 | 3.34 ± 0.44 | 0.06 |
| 16a | 2-Cl-Ph | H | 56.3 | 3.41 ± 0.36 | 0.20 [38] |
| 16b | 3-Cl-Ph | H | 91.9 | 3.93 ± 0.37 | 0.37 |
| 16c | 4-Cl-Ph | H | 147.6 | 3.89 ± 0.36 | 0.23 |
| 17a | 2-Br-Ph | H | 92.2 | 3.58 ± 0.44 | 0.21 [38] |
| 17b | 3-Br-Ph | H | 409.0 | 4.10 ± 0.44 | 0.39 |
| 17c | 4-Br-Ph | H | 307.9 | 4.06 ± 0.44 | 0.23 |
| 18a | 2-CF3-Ph | H | 109.4 | 4.09 ± 0.42 | NF |
| 18b | 3-CF3-Ph | H | 329.5 | 4.25 ± 0.42 | 0.43 |
| 18c | 4-CF3-Ph | H | ND | 3.91 ± 0.41 | 0.74 |
| 19a | 2-NO2-Ph | H | ND | 3.15 ± 0.38 | 0.80 [38] |
| 19b | 3-NO2-Ph | H | ND | 3.28 ± 0.38 | 0.71 |
| 19c | 4-NO2-Ph | H | ND | 3.36 ± 0.38 | 1.26 |
| DCMU | – | – | 1.9 | – | – |
