Table 2.
Acetalization of fullerene epoxide 1a with carbonyl compound in the presence of an acid catalyst.
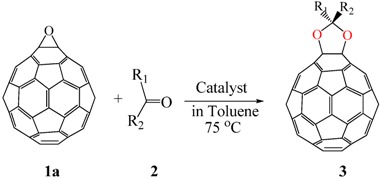 | ||||
|---|---|---|---|---|
| Entry | 2 | Catalyst (equiv./amount) | Reaction Time | Yield a of 3 / % |
| 1 |
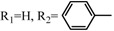
|
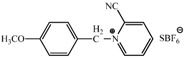 4a (0.29) 4a (0.29) |
60 min | 92 |
| 2 | 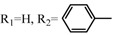 |
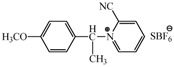 4b (0.28) 4b (0.28) |
90 min | 91 |
| 3 | 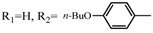 |
4a (0.29) | 60 min | 95 |
| 4 | 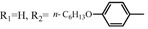 |
4b (0.28) | 4.5 h | 88 |
| 5 | 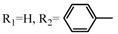 |
BF3·Et2O (one drop) | 60 min | 89 |
| 6 | 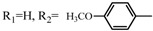 |
Amberlyst 15® (268 mg/0.02 mmol) | 3 h | 96 |
| 7 | 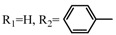 |
Montmorillonite (250 mg/0.02 mmol) | 4 h reflux | 60 |
| 8 | 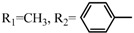 |
4a (0.27) | 30 min | 45 |
| 9 | R1=CH3, R2=C2H5 | 4a (0.27) | 30 min | 44 |
| 10 | Cyclohexanone | Amberlyst 15® (250 mg/0.02 mmol) | 65 °C, 5 h in benzene | 60 |
| 11 | γ-Butyrolactone | BF3·Et2O (one drop) | 3 h in benzene | 75 |
a Isolated yield.
