Abstract
A novel series of 3-(2-furyl)acrylate monosaccharide esters Ia–f and menthyloxycarbonyl monosaccharide esters IIa–f were designed and synthesized. The chemical structures of the target compounds were confirmed by IR, 1H- and 13C-NMR and ESI-MS, and the target compounds were investigated for their in vitro antibacterial and antifungal activities. The antibacterial screening results showed that the 3-(2-furyl)acrylate monosaccharide ester derivatives Ia–f were either inactive or only weakly active against the three Gram-positive bacterial strains tested, whereas the menthyloxycarbonyl monosaccharide ester derivatives IIa–f exhibited higher levels of activity, with compound IIe being especially potent. The results of the antifungal screening revealed that compounds Ib, Ie, IIb and IIc displayed potent in vitro activities, whereas If and IIf showed promising activities against all of the microorganisms tested, with If exhibiting levels of activity deserving of further investigation.
Keywords: carbohydrate-acetonides, 3-(2-furyl)acrylate monosaccharide esters, menthyloxycarbonyl monosaccharide esters, antibacterial activities, antifungal activities
1. Introduction
Microbial food contamination problems have been the cause of much public concern over the last few decades because of an increase in the number of infections and diseases originating from the consumption of spoiled food [1]. Antibacterial and antifungal agents are necessary for food preservation, especially for food processors, because bacterial and fungal growth are important causes of food spoilage. For this reason, many investigators have focused their research efforts on finding new efficient, low toxicity and environmentally friendly antibacterial and antifungal agents.
Sugar esters have been widely used as cosmetic and pharmaceutical industries for many years because they are considered biocompatible, biodegradable, and nontoxic and can be synthesized from renewable resources [2,3,4,5,6,7]. Furthermore, sugar esters have attracted considerable research interest in recent years because they have exhibited a variety of biological activities, including insecticidal [8], antitumor [9,10,11,12,13], antimicrobial and antifungal properties [14,15,16,17,18]. These results prompted us to design and synthesize a novel series of sugar esters in the hope that we might find some promising antimicrobial or antifungal agents. From a review of the literature, there have been many reports concerning propyl 3-(2-furanyl)acrylate ester and (−)-menthol and their applications in the food, beverage and cosmetics industries. Furthermore, 3-(2-furyl)acrylic acid and its ester derivatives and (−)-menthol have also been reported as antimicrobial agents [19,20,21,22,23]. But these compounds have poor solubility in water and thus result in low bioactivity. Inspired by these observations, we planned to couple 3-(2-furyl) acrylic acid or (−)-menthol with monosaccharides to obtain the corresponding water-soluble monosaccharide esters, as it was envisaged that these novel compounds would combine the favorable properties of sugars with either the 3-(2-furyl)acrylic acid esters or (−)-menthol and will be more bioavailable.
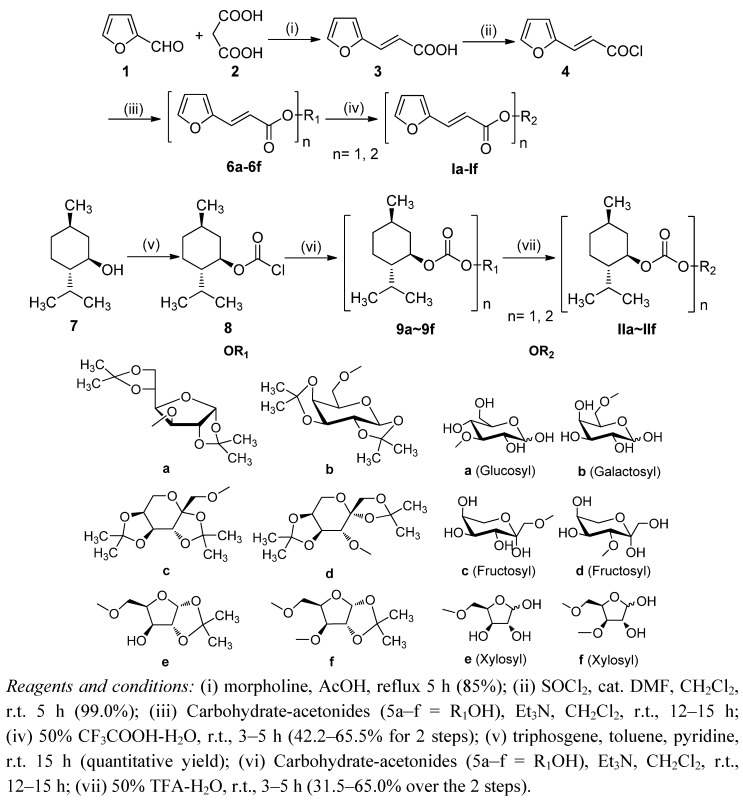
Based upon the aforementioned considerations, herein we describe the synthesis of two novel series, including a series of 3-(2-furyl) acrylate monosaccharide esters Ia–f and a series of menthyloxycarbonyl monosaccharide esters IIa–f (Scheme 1).
Scheme 1.
Synthesis of compounds Ia–f and IIa–f.
Furthermore, their in vitro antibacterial activities against Bacillus subtilis, Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli and Pseudomonas aeruginosa, and antifungal activities against Aspergillus flavus, Aspergillus niger, Aspergillus fumigatus and Geotrichum candidum were investigated.
2. Results and Discussion
2.1. Chemical Synthesis
The target compounds Ia–f and IIa–f were synthesized according to conventional procedures as outlined in Scheme 1. Compound 3 [24] was prepared by a modified procedure which provided the product more conveniently and in higher yield, and was subsequently reacted with thionyl chloride to afford α-furanacryloyl chloride 4. Compounds 6a–f were obtained via the esterification of intermediate 4 with the corresponding one anomer of O-isopropylidene-protected monosaccharides 5a–f in dry dichloromethane in the presence of Et3N at room temperature. Subsequent deprotection of the O-isopropylidenes with a 50% TFA-H2O solution at room temperature gave the desired 3-(2-furyl)acrylate monosaccharide esters Ia–f as a mixture of anomers for Ia, Ib, Ie and If due to mutarotation [25]. The menthyloxycarbonyl monosaccharide esters IIa–f were synthesized from the readily available alcohol (−)-menthol (7), which was reacted with triphosgene and pyridine in toluene to give menthyl chloroformate 8 in quantitative yield [26]. Chloroformate 8 was taken into the next step without purification and reacted with the one anomer of O-isopropylidene protected monosaccharides 5a–f in dry dichloromethane in the presence of Et3N at room temperature to afford the corresponding menthol carbonates 9a–f in high yields. Subsequent deprotection of the O-isopropylidenes with a 50% TFA-H2O solution at room temperature gave the desired anomeric mixture of menthyloxycarbonyl monosaccharide esters IIa–f. Among them, IIa, IIb, IIe and IIf were obtained as a mixture of anomers due to mutarotation. All of the target compounds were purified by silica gel flash column chromatography, and their chemical structures were confirmed by IR, 1H- and 13C-NMR and ESI-MS. To the best of our knowledge, none of these monosaccharide esters have been reported in the literature, and therefore represent novel compounds.
2.2. Antibacterial and Antifungal Activities
2.2.1. Antibacterial Activities
The in vitro antibacterial activities of the monosaccharide esters Ia–f and IIa–f were tested against five bacterial strains, including the three Gram-positive organisms, B. subtilis, S. aureus, and S. epidermidis, and the two Gram-negative organisms E. coli and P. aeruginosa. The antibacterial assays were conducted according to the NCCLS (National Committee for Clinical Laboratory Standards) document M100-S12 method [27]. Standard antibacterial agents, including penicillin and streptomycin, were also screened under identical conditions for the sake of comparison. The minimum inhibitory concentrations (MIC) values of the tested compounds are shown in Table 1.
Table 1.
MIC values (µg/mL) of the tested compounds against selected bacterial strains.
| Compound | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | ||||
| B. subtilis | S. aureus | S. epidermidis | E. coli | P. aeruginosa | |
| Ia | 32 | 32 | 32 | >64 | >64 |
| Ib | 32 | 32 | 32 | >64 | >64 |
| Ic | >64 | 32 | >64 | >64 | >64 |
| Id | >64 | 32 | >64 | >64 | >64 |
| Ie | 32 | 32 | >64 | >64 | >64 |
| If | 32 | >64 | >64 | >64 | >64 |
| IIa | 32 | 16 | 32 | >64 | >64 |
| IIb | 8 | 32 | 32 | >64 | >64 |
| IIc | 32 | 32 | 16 | >64 | >64 |
| IId | 32 | 32 | 32 | >64 | >64 |
| IIe | 2 | 2 | 8 | >64 | >64 |
| IIf | 8 | 16 | 16 | >64 | >64 |
| 3 | 8 | 32 | 16 | >64 | >64 |
| (−)-Menthol | 8 | 16 | 8 | 8 | 4 |
| Penicillin | 1 | 1 | 4 | 32 | 16 |
| Streptomycin | 8 | 8 | 16 | 4 | 2 |
Negative control 5% DMSO---no activity.
The compounds tested clearly exhibited varying degrees of antibacterial activity. The 3-(2-furyl)acrylate monosaccharide ester derivatives Ia–f were either inactive or only weakly active against the three Gram-positive bacterial strains tested, whereas the menthyloxycarbonyl monosaccharide ester derivatives IIa–f showed greater levels of activity, with compound IIe exhibiting remarkably high levels of antibacterial activities against all three bacterial strains. It was also shown that all of the target compounds were inactive against the gram-negative bacterial strains tested.
2.2.2. Antifungal Activities
The in vitro antifungal activities of the monosaccharide esters Ia–f and IIa–f were determined against four fungal strains, including A. flavus, A. niger, A. fumigatus, and G. candidum, using clotrimazole as a reference standard. The antifungal activity assays were conducted according to the NCCLS standard M27-A method [28]. The MIC data of the tested compounds are presented in Table 2.
Table 2.
MIC data (µg/mL) of the tested compounds against selected fungal strains.
| Compound | MIC (µg/mL) | |||
|---|---|---|---|---|
| A. flavus | A. niger | A. fumigatus | G. candidum | |
| Ia | 32 | 32 | 32 | 32 |
| Ib | 16 | 16 | 8 | 32 |
| Ic | 32 | >64 | >64 | >64 |
| Id | >64 | >64 | 32 | >64 |
| Ie | 16 | 16 | 8 | 32 |
| If | 2 | 2 | 2 | 4 |
| IIa | >64 | >64 | >64 | >64 |
| IIb | 16 | 16 | 8 | 32 |
| IIc | 16 | 32 | 8 | 16 |
| IId | >64 | >64 | >64 | >64 |
| IIe | 32 | >64 | >64 | 32 |
| IIf | 4 | 4 | 4 | 8 |
| 3 | 32 | >64 | >64 | >64 |
| (−)-Menthol | 32 | 32 | 32 | >64 |
| Clotrimazole | 8 | 8 | 4 | 16 |
Negative control 5% DMSO---no activity.
The in vitro antifungal activity results showed that compounds If and IIf exhibited potent activities against all of the microorganisms tested. Furthermore, both of these compounds, especially If, exhibited activities comparable to those of the standard fungicide, clotrimazole, indicating that these compounds are worthy of further investigation. It was also found that compounds Ib, Ie, IIb and IIc showed potent in vitro antifungal activities, and that their activities were superior to those of the corresponding 3-(2-furyl)acrylic acid (3) and (−)-menthol. The other monosaccharide esters Ia, Ic, Id, IIa, IId and IIe showed no activity against the antifungal strains tested.
3. Experimental
3.1. Materials and Reagents
Melting points were determined in open glass capillaries using a paraffin bath and are uncorrected. 1H- and 13C-NMR spectra were measured on a Varian INOVA-400 instrument at 400 and 100 MHz, respectively, using TMS as an internal standard in CDCl3 or D2O solvents. IR spectra were obtained on a Thermo Nicolet AVATAR 370 FT-IR instrument using KBr plates. Mass spectra were recorded on a Waters Q-TOF Premier mass spectrometer. Optical rotation data were collected after mutarotation equilibration in about 10 min on a Perkin-Elmer 341 Polarimeter using HPLC grade anhydrous MeOH. All commercially available reagents and anhydrous solvents were purchased at the highest commercial quality and were used without further purification. The bacterial and fungal strains were obtained from Sichuan Industrial Institute of Antibiotics, Sinopharm Group Co. Ltd., (SINOPHARM-SIIA, Chengdu, China).
3.2. Chemical Synthesis
3.2.1. Preparation of 3-(2-furyl)acrylic acid (3)
Furfural (28.8 g, 0.30 mol) was dissolved in AcOH (120 mL) and morpholine (26.1 g, 0.30 mol) was slowly added to the stirred mixture at 25 °C over a period of 10 min. Malonic acid (31.2 g, 0.30 mol) was then added to the reaction mixture and the resulting mixture was heated at reflux for 5.0 h. The mixture was then cooled to 25 °C and poured into cold water (400 mL). The resulting precipitate was collected by filtration, washed with water, and dried under vacuum at 40 °C in a vacuum oven to give the desired product. Yield: 35.2 g (85%), mp 140–141 °C (lit. m.p. 140–141 °C [24]).
3.2.2. Preparation of 3-(2-furyl)acryloyl chloride (4)
Thionyl chloride (17.85 g, 0.15 mol) was added to a stirred solution of 3-(2-furyl)acrylic acid (3, 13.8 g, 0.1 mol) in CH2Cl2 (70 mL) at room temperature and the resulting mixture was heated at reflux for 3 h. The mixture was then cooled and concentrated in vacuo to afford 3-(2-furyl)acryloyl chloride quantitatively, which was used directly without further purification in the following reaction.
3.2.3. A general procedure for the synthesis of compounds Ia–f
A solution of 3-(2-furyl)acryloyl chloride (4, 0.024 mol or 0.048 mol in the synthesis of 6f) in dry CH2Cl2 (15 mL) was added in a dropwise manner to a solution of carbohydrate-acetonide 5a–f (only one anomer was used, 0.02 mol) and Et3N (0.05 mol) in dry CH2Cl2 (60 mL) at 0 °C in an ice bath. The resulting solution was allowed to warm to room temperature and stirred for 12–15 h. The reaction mixture was then diluted with CH2Cl2 (40 mL) and washed sequentially with H2O (2 × 25 mL), saturated aqueous sodium bicarbonate (25 mL) and saturated aqueous sodium chloride (25 mL) and dried (Na2SO4). The solvent was then removed in vacuo and to give the crude products 6a–f (only one anomer was obtained), which were used directly in the next steps without further purification. A 50% TFA-H2O solution (30 mL) was added to each of the compounds 6a–f and the mixture was continuously stirred at room temperature for 3–5 h. Upon completion of the reaction (TLC, developing solvent: CHCl3/CH3OH = 15:1), the solvents were removed in vacuo and residue was purified by silica gel flash column chromatography (eluent: CHCl3/CH3OH = 10:1) to give the compounds Ia–f as light yellow foams.
3-O-[3-(2-Furyl)acryloyl]-α/β-D-glucopyranose (Ia): yield 48.5%, (α:β isomer = 2:3 based on 1H-NMR); [α]D20= +30.1 (c = 0.5, CH3OH); IR (KBr, cm−1): 3405.65, 2936.51, 1688.63, 1635.15, 1390.73, 1312.25, 1282.29, 1264.77, 1211.67, 1174.19, 1080.92, 1020.63, 751.60; 1H-NMR (D2O): δ 7.71 (s, 1H, Ar-H), 7.65 (d, J = 16 Hz, 1H, =CH), 6.90 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H), 6.46 (d, J = 16 Hz, 1H, =CH), 5.34 (d, J = 4.0 Hz, 0.4H, 1'-Hα), 5.31 (d, J = 10.0 Hz, 0.6H, 1'-Hβ), 5.13 (t, J = 9.6 Hz, 0.6H, 3'-Hβ), 4.82 (t, J = 8.6 Hz, 0.6H, 3'-Hα), 4.00–3.92 (m, 1H, 2'-H), 3.89–3.78 (m, 2H, 6'-H), 3.75–3.70 (m, 1H, 5'-H), 3.66–3.50 (m, 1H, 4'-H); 13C-NMR (D2O): δ 166.49, 166.30, 147.86, 143.49, 130.44, 130.33, 114.13, 111.38, 111.24, 110.27, 93.36, 89.59, 74.93, 73.26, 72.85, 70.03, 69.97, 68.74, 67.43, 65.47, 58.07, 57.90; ESI-MS (m/z): 623.5 [2M+Na]+, 339.05 [M+K]+, 323.10 [M+Na]+.
6-O-[3-(2-Furyl)acryloyl]-α/β-D-galactopyranose (Ib): yield 65.5%, (α:β isomer = 1:3 based on 1H-NMR); [α]D20= +33.2 (c = 0.5, CH3OH); IR (KBr, cm−1): 3421.48, 2921.46, 1689.56, 1646.43, 1484.23, 1311.05, 1287.52, 1267.55, 1219.03, 1178.91, 1068.70, 1028.97, 766.04; 1H-NMR (D2O): δ 7.70 (s, 1H, Ar-H), 7.60 (d, J = 16 Hz, 1H, =CH), 6.90 (s, 1H, Ar-H), 6.65 (s, 1H, Ar-H), 6.42 (d, J = 16 Hz, 1H, =CH), 5.33 (d, J = 4.0 Hz, 0.33H, 1'-Hα), 4.65 (d, J = 8.0 Hz, 0.67H, 1'-Hβ), 4.47–4.36 (m, 2H, 2'-H, 3'-H), 4.10–4.01 (m, 2H, 6'-H), 3.95–3.86 (m, 1H, 5'-H), 3.73–3.54 (m, 1H, 4'-H); 13C-NMR (D2O): δ 171.05, 152.68, 148.31, 135.04, 118.96, 116.04, 115.16, 98.90, 94.83, 78.0, 74.97, 74.90, 74.12, 71.40, 71.19, 70.66, 66.23, 63.2, 62.5; ESI-MS (m/z): 623.18 [2M+Na]+, 339.06 [M+K]+, 323.10 [M+Na]+.
1-O-[3-(2-Furyl)acryloyl]-β-D-fructopyranose (Ic): yield 51.5%; [α]D20= −16.8 (c = 0.5, CH3OH); IR (KBr, cm−1): 3425.36, 2919.49, 1689.56, 1633.87, 1392.49, 1309.13, 1280.21, 1268.82, 1212.08, 1165.22, 1079.39, 751.26; 1H-NMR (D2O): δ 7.69 (s, 1H, Ar-H), 7.62 (d, J = 16 Hz, 1H, =CH), 6.90 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.43 (d, J = 16 Hz, 1H, =CH), 4.35(d, J = 11.6 Hz, 1H, 1'-H), 4.33 (d, J = 11.6 Hz, 1H, 1'-H), 4.12–4.06 (m, 2H, 4'-H+3'-H), 3.99–3.96 (m, 1H, 5'-H), 3.88–3.86 (m, 1H, 6'-H), 3.77–3.74 (m, 1H, 6'-H); 13C-NMR (D2O): δ 171.12, 152.79, 148.44, 135.40, 119.14, 115.94, 115.20, 99.62, 71.83, 71.46, 70.51, 68.19, 66.10; ESI-MS (m/z): 623.58 [2M+Na]+, 339.28 [M+K]+, 323.34 [M+Na]+.
3-O-[3-(2-Furyl)acryloyl]-β-D-fructopyranose (Id): yield 42.2%; [α]D20= −15.0 (c = 0.5, CH3OH); IR (KBr, cm−1): 3423.50, 2919.06, 1690.32, 1639.41, 1390.12, 1311.75, 1285.37, 1267.76, 1216.13, 1175.78, 1075.620, 1048.93, 750.55; 1H-NMR (D2O): δ 7.71 (s, 1H, Ar-H), 7.66 (d, J = 16 Hz, 1H, =CH), 6.92 (s, 1H, Ar-H), 6.65 (s, 1H, Ar-H), 6.42 (d, J = 16 Hz, 1H, =CH), 5.34 (d, J = 10.0 Hz, 1H, 3'-H), 4.18 (d, J = 10.8 Hz, 2H, 6'-H), 4.13 (s, 2H, 1'-H), 3.91–3.78 (m, 1H, 4'-H), 3.65–3.49 (m, 1H, 3'-H); 13C-NMR (D2O): δ 170.93, 152.77, 148.52, 135.64, 119.29, 115.79, 115.22, 99.64, 72.23, 71.69, 70.63, 66.43, 65.99; ESI-MS (m/z): 339.28 [M+K]+, 323.34 [M+Na]+.
5-O-[3-(2-Furyl)acryloyl]-α/β-D-xylofuranose (Ie): yield 54.8%, (α:β isomer = 3:1 based on 1H-NMR); [α]D20= +15.6 (c = 0.5, CH3OH); IR (KBr, cm−1): 3418.32, 2921.25, 1695.67, 1482.55, 1348.08, 1304.12, 1266.78, 1209.46, 1068.42, 1033.91, 752.10; 1H-NMR (D2O): δ 7.69 (s, 1H, Ar-H), 7.63 (d, J = 16 Hz, 1H, =CH), 6.89 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.43 (d, J = 16 Hz, 1H, =CH), 5.29 (d, J = 2.8 Hz, 0.75H, 1'-Hα), 5.26–5.03 (m, 1H, 4'-H), 4.74 (d, J = 8.0 Hz, 0.25H, 1'-Hβ), 4.06–3.86 (m, 2H, 5'-H), 3.84–3.80 (m, 1H, 3'-H), 3.53–3.45 (m, 1H, 2'-H); 13C-NMR (D2O): δ 169.35, 151.66, 147.22, 132.54, 116.26, 114.84, 112.45, 99.72, 96.68, 77.66, 77.05, 76.12, 75.39, 73.96, 73.80, 65.55, 63.23; ESI-MS (m/z): 563.48 [2M+Na]+, 309.30 [M+K]+, 293.28 [M+Na]+.
3,5-Bi-O-[3-(2-furyl)acryloyl]-α/β-D-xylofuranose (If): yield 63.6%, (α:β isomer = 6:1 based on 1H-NMR); [α]D20= −15.4 (c = 0.5, CH3OH); IR (KBr, cm−1): 3417.95, 2921.02, 1703.85, 1638.68, 1479.26, 1351.47, 1308.86, 1264.24, 1210.32, 1168.12, 1077.95, 1045.66, 748.99; 1H-NMR (CDCl3): δ 7.50 (d, J = 2.0 Hz, 1H, Ar-H), 7.47 (d, J = 1.6 Hz, 1H, Ar-H), 7.44 (d, J = 16.0 Hz, 1H, =CH), 7.43 (d, J = 16.0 Hz, 1H, =CH), 6.65 (d, J = 3.2 Hz, 1H, Ar-H), 6.61 (d, J = 3.2 Hz, 1H, Ar-H), 6.48 (dd, J = 3.2, 1.6 Hz, 1H, Ar-H), 6.46 (dd, J = 3.2, 2.0 Hz, 1H, Ar-H), 6.32 (d, J = 16.0 Hz, 1H, =CH), 6.30 (d, J = 16.0 Hz, 1H, =CH), 5.57 (d, J = 4.4 Hz, 0.85H, 1'-Hα), 5.37 (d, J = 8.4 Hz, 0.15H, 1'-Hβ), 5.35–5.28 (m, 1H, 2'-H), 4.76–4.70 (m, 1H, 4'-H), 4.40–4.35 (m, 2H, 5'-H), 4.34–4.29 (m, 1H, 3'-H); 13C-NMR (CDCl3): δ 166.84, 166.75, 150.66, 150.44, 145.27, 144.92, 132.69, 132.61, 131.88, 131.78, 115.98, 115.30, 115.24, 114.86, 113.97, 112.45, 112.29, 102.98, 96.08, 78.66, 77.35, 77.03, 76.72, 75.39, 74.98, 63.80, 62.47; ESI-MS (m/z): 803.25 [2M+Na]+, 429.12 [M+K]+, 413.14 [M+Na]+.
3.2.4. Preparation of Menthyl Chloroformate 8
A solution of pyridine (21.9 mL, 0.27 mmol) in toluene (150 mL) was added in a dropwise manner to a stirred solution of triphosgene (21.96 g, 0.074 mol) in toluene (260 mL) at 0 °C under an argon atmosphere. Stirring was continued for 15 min at 0 °C and a solution of (−)-menthol (7, 28.12 g, 0.18 mol) in toluene (100 mL) was then slowly added through a dropping funnel. The reaction mixture was allowed to warm to ambient temperature and stirred for 15 h. The reaction mixture was then diluted with water (300 mL) and extracted with toluene (2 × 200 mL). The combined organics were washed sequentially with water (200 mL) and brine (200 mL) and dried (Na2SO4). The solvent was removed in vacuo to give the title compound 8 as colorless oil (39.3 g, quant.), which was used directly in the next step without further purification.
3.2.5. A General Procedure for the Synthesis of Compounds IIa–f
A solution of menthyl chloroformate 8 (0.024 mol or 0.05 mol for the synthesis of 9f) in dry CH2Cl2 (15 mL) was added in a drop-wise manner to a solution of carbohydrate-acetonide 5a–f (only one anomer was used, 0.02 mol) and Et3N (0.05 mol) in dry CH2Cl2 (60 mL) at 0 °C in an ice bath. The resulting solution was allowed to warm to room temperature and stirred for 12–15 h. The reaction mixture was then diluted with CH2Cl2 (40 mL) and washed sequentially with H2O (2 × 25 mL), saturated aqueous sodium bicarbonate (25 mL) and saturated aqueous sodium chloride (25 mL) before being dried (Na2SO4). The solvent was removed in vacuo to give a crude product from 9a–f (only one anomer was obtained), which was used directly in the next step without further purification. A 50% TFA-H2O solution (30 mL) was added to one of the compounds 9a–f and the resulting mixture continuously stirred at room temperature for 3–5 h. Upon completion of the reaction (TLC, developing solvent: CHCl3/CH3OH = 20:1), the solvents were removed in vacuo and the crude residue was purified by silica gel flash column chromatography (eluent: CHCl3/CH3OH = 30:1) to give the compounds IIa–f as a colorless foam.
3-O-Menthyloxycarbonyl-α/β-D-glucopyranose (IIa): yield 60.0%, (α:β isomer = 1:1 based on 1H-NMR); [α]D20 = +5.6 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ 5.36–5.30 (m, 0.5H, 1'-Hβ), 5.00–4.90 (m, 0.5H, 1'-Hα), 4.82–4.72 (m, 1H, 3'-H), 4.58–4.48 (m, 1H, 5'-H), 4.05–3.92 (m, 2H, 4'-H+CHO), 3.83–3.72 (m, 1H, 6'-H), 3.70–3.44 (m, 2H, 6'-H+2'-H), 2.10–2.02 (m, 1H, CH), 2.01–1.92 (m, 1H, CH), 1.72–1.62 (m, 2H, CH2), 1.54–1.38 (m, 2H, CH+CH2), 1.12–0.99 (m, 2H, CH2), 0.91 (d, J = 6.4 Hz, 3H, CH3), 0.88 (d, J = 6.4 Hz, 3H, CH3), 0.88–0.84 (m, 1H, CH2), 0.76 (d, J = 4.4 Hz, 3H, CH3); 13C-NMR (CDCl3): δ 156.10, 92.30, 88.45, 79.52, 73.55, 73.08, 72.80, 71.05, 70.66, 69.25, 68.82, 62.02, 58.40, 57.96, 47.02, 40.48, 34.02, 31.35, 25.94, 23.24, 22.00, 20.68, 16.21; ESI-MS (m/z): 401.28 [M+K]+, 385.30 [M+Na]+.
6-O-Menthyloxycarbonyl-α/β-D-galactopyranose (IIb): yield 65.0%, (α:β isomer = 1:4 based on 1H-NMR), [α]D20 = −9.9 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ 5.38 (d, J = 3.6 Hz, 0.2H, 1'-Hα), 4.64 (d, J = 8.4 Hz, 0.8H, 1'-Hβ), 4.63–4.47 (m, 1H, 6'-H), 4.38–4.06 (m, 2H, 2'-H+6'-H), 4.05–3.95 (m, 2H, 3'-H+CHO), 3.82–3.75 (m, 1H, 5'-H), 3.73–3.62 (m, 1H, 4'-H), 2.07–2.04 (m, 1H, CH), 2.01–1.92 (m, 1H, CH), 1.72–1.63 (m, 2H, CH2), 1.52–1.34 (m, 2H, CH+CH2), 1.10–0.99 (m, 2H, CH2), 0.91 (d, J = 6.0 Hz, 3H, CH3), 0.88 (d, J = 6.8 Hz, 3H, CH3), 0.86-0.84 (m, 1H, CH2), 0.76 (d, J = 6.8 Hz, 3H, CH3); 13C-NMR (CDCl3): δ 154.89, 96.88, 92.52, 78.97, 73.10, 72.74, 72.28, 69.60, 69.06, 68.78, 67.30, 66.90, 66.23, 66.02, 46.90, 40.60, 34.02, 31.34, 25.84, 23.14, 21.97, 20.76, 16.16; ESI-MS (m/z): 401.31 [M+K]+, 385.29 [M+Na]+.
1-O-Menthyloxycarbonyl-β-D-fructopyranose (IIc): yield 60.0%; [α]D20= −44.8 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ 4.54 (d, J = 11.2 Hz, 1H, 1'-H), 4.52 (d, J = 11.6 Hz, 1H, 1'-H), 4.34–4.25 (m, 2H, 4'-H+3'-H), 4.14–4.06 (m, 1H, 5'-H), 4.05–4.00 (m, 1H, CHO), 3.92–3.82 (m, 1H, 6'-H), 3.80–3.73 (m, 1H, 6'-H), 2.07–2.03 (m, 1H, CH), 2.00–1.90 (m, 1H, CH), 1.72–1.64 (m, 2H, CH2), 1.52–1.36 (m, 2H, CH+CH2), 1.10–0.99 (m, 2H, CH2), 0.91 (d, J = 7.2 Hz, 3H, CH3), 0.88 (d, J = 7.2 Hz, 3H, CH3), 0.86–0.84 (m, 1H, CH2), 0.76 (d, J = 7.2 Hz, 3H, CH3); 13C-NMR (CDCl3): δ 155.03, 96.81, 79.32, 73.39, 70.31, 69.25, 67.51, 63.57, 46.92, 40.54, 33.99, 31.34, 25.86, 23.13, 21.96, 20.73, 16.13; ESI-MS (m/z): 401.29 [M+K]+, 385.29 [M+Na]+.
3-O-Menthyloxycarbonyl-β-D-fructopyranose (IId): yield 53.4%; [α]D20= −61.3 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ 4.89 (d, J = 9.6 Hz, 1H, 3'-H), 4.59–4.51 (m, 1H, 6'-H), 4.15–4.10 (m, 1H, 6'-H), 4.06 (s, 2H, 1'-H), 3.88–3.82 (m, 1H, CHO), 3.67–3.63 (m, 1H, 4'-H), 3.52–3.45 (m, 1H, 3'-H), 2.10–2.03 (m, 1H, CH), 1.98–1.88 (m, 1H, CH), 1.70–1.67 (m, 2H, CH2), 1.54–1.36 (m, 2H, CH+CH2), 1.14–0.99 (m, 2H, CH2), 0.91 (d, J = 7.2 Hz, 3H, CH3), 0.88 (d, J = 7.2 Hz, 3H, CH3), 0.87–0.85 (m, 1H, CH2), 0.76 (d, J = 7.8 Hz, 3H, CH3); 13C-NMR (CDCl3): δ 155.67, 97.06, 79.59, 73.90, 69.79, 68.67, 64.74, 63.16, 46.90, 40.46, 33.97, 31.35, 26.12, 23.23, 21.95, 20.66, 16.18; ESI-MS (m/z): 401.30 [M+K]+, 385.31 [M+Na]+.
5-O-Menthyloxycarbonyl-α/β-D-xylofuranose (IIe): yield 42.0%, (α:β isomer = 4:1 based on 1H-NMR); [α]D20= −29.7 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ5.53 (d, J = 2.0 Hz, 0.8H, 1'-Hα), 4.58–4.48 (m, 1.2H, 1'-Hβ+4'-H), 4.46–4.38 (m, 2H, 3'-H+5'-H), 4.36–4.24 (m, 2H, 2'-H+5'-H), 4.20–4.13 (m, 1H, CHO), 2.10–2.03 (m, 1H, CH), 1.98–1.90 (m, 1H, CH), 1.72–1.64 (m, 2H, CH2), 1.53–1.36 (m, 2H, CH+CH2), 1.10–0.99 (m, 2H, CH2), 0.92 (d, J = 7.2 Hz, 3H, CH3), 0.89 (d, J = 6.8 Hz, 3H, CH3), 0.87–0.85 (m, 1H, CH2), 0.77 (d, J = 6.8 Hz, 3H, CH3); 13C-NMR (CDCl3): δ 155.21, 155.06, 102.80, 96.09, 80.15, 79.83, 79.19, 79.10, 76.36, 75.70, 75.47, 69.85, 67.40, 66.45, 65.96, 46.83, 40.54, 33.94, 31.31, 25.86, 23.12, 21.92, 20.68, 16.13; ESI-MS (m/z): 371.27 [M+K]+, 355.31 [M+Na]+.
3,5-Di-O-menthyloxycarbonyl-α/β-D-xylofuranose (IIf): yield 31.5%, (α:β isomer = 3:1 based on 1H-NMR); [α]D20= −40.2 (c = 1.0, CH3OH); 1H-NMR (CDCl3): δ5.21 (d, J = 3.6 Hz, 0.75H, 1'-Hα), 5.02 (d, J = 8.4 Hz, 0.25H, 1'-Hβ), 4.61–4.47 (m, 3H, 4'-H+5'-H+3'-H), 4.38–4.29 (m, 1H, 2'-H), 4.29–4.14 (m, 2H, 5'-H+CHO), 4.14–3.96 (m, 1H, CHO), 2.09-2.03 (m, 2H, 2CH), 1.98–1.91 (m, 2H, 2CH), 1.72–1.57 (m, 4H, 2CH2), 1.53–1.36 (m, 4H, 2CH+2CH2), 1.16–0.99 (m, 4H, 2CH2), 0.96–0.85 (m, 16H, 4CH3+2CH2), 0.84–0.75 (m, 6H, 2CH3); 13C-NMR (CDCl3) δ: 155.66, 155.62, 101.45, 96.35, 79.75, 79.60, 79.12, 78.03, 77.16, 76.58, 75.30, 73.67, 63.72, 63.25, 46.85, 40.58, 33.92, 31.38, 26.32, 23.20, 21.95, 20.67, 16.20; ESI-MS (m/z): 553.21 [M+K]+, 537.44 [M+Na]+, 493.49 [M-CO2+Na]+.
3.3. Antibacterial and Antifungal Activity Assays
For each tested compound, 1.28 mg sample was put in a 25 mL flask and dissolved in 10 mL 5% DMSO. The drug was filtered into autoclaving centrifuge tubes in super clean bench. Penicillin, streptomycin and clotrimazole solutions were prepared to the same concentration as the positive control drug. After all bacteria and fungi were recovered, each single colony was picked and inoculated in 3 mL of either a sterilized Mueller-Hinton (MH) broth cultured at 37 °C for 18–24 h (for bacteria) or a Sabouraud Dextrose (SD) broth cultured at 28 °C for 48 h (for fungi). The microorganism solution was corrected to 0.5 McFarland standard turbidity using either the MH or SD broths, and subsequently diluted by 1:100 (amount of microorganism approximately 106 colony forming units/mL) with either the MH or SD broth and inoculated immediately. Blank broth (100 µL) was added into all the wells of rows 2–12 of a 96-well plate. Broth (180 µL) and dispensed drug liquor (20 µL) where added to the first row of the 96-well plate and the mixture was well mixed. A sample of the resulting mixture (100 µL) was inhaled and added into the corresponding wells of the second row. The same mixing and transferring operations were repeated until the wells in the 12th row were filled. The concentrations of drug in the wells of rows 1–12 were 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.0625 µg/mL, respectively. The seventh and eighth rows of the 96-well plate were used for the positive and negative controls, respectively. A diluted microorganism solution (100 µL) was added to each well of the 96-well plate and the plate was shaken on a shaker instrument and subsequently stored in an incubator for 24–48 h at 37 °C (for bacteria) and at 28 °C (for fungi). Each experiment was performed in triplicate.
4. Conclusions
In summary, the design and synthesis of two novel series of 3-(2-furyl) acrylate monosaccharide esters Ia–f and menthyloxycarbonyl monosaccharide esters IIa–f from simple starting materials and under mild reaction conditions has been reported. The antibacterial and antifungal properties of these novel compounds were evaluated. The results revealed that the 3-(2-furyl)acrylate monosaccharide ester derivatives Ia–f were either inactive or only weakly active against the three Gram-positive bacterial strains tested (B. subtilis, S. aureus, S. epidermidis), whereas the menthyloxy carbonyl monosaccharide ester derivatives IIa–f exhibited greater degrees of activity, with compound IIe showing remarkably high antibacterial activity. Compound Ib, Ie, IIb and IIc displayed potent in vitro antifungal activities, whereas If and IIf showed promising antifungal activities against all of the microorganisms tested, with compound If exhibiting significantly high activities, which are worthy of further investigation.
Acknowledgments
This work was financially supported by the National Science and Technology Major Project on “Key New Drug Creation and Manufacturing Program” (2009ZX09103-037), the Chinese National Natural Science Foundation (20872099), the Research Program of Sichuan Science and Technology Agency (2008JY0142), and the related Post-Doctoral Research Program.
Conflict of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds Ia–f and IIa–f are available from the authors.
References
- 1.Wilson C.L. Microbial Food Contamination. 2nd. CRC Press, Taylor & Francis Group, LLC; Boca Raton, FL, USA: 2007. [Google Scholar]
- 2.Louis B.B.C. A Sugar Ester Process and Its Applications in Calf Feeding and Human Food Additives. In: Hickson J., editor. Sucrochemistry. Chapter 8. American Chemical Society; Washington, DC, USA: 1977. pp. 115–120. [Google Scholar]
- 3.Garti N., Aaerin A., Fanun M. Non-ionic sucrose esters microemulsions for food applications. Colloids Surf. A Physicochem. Eng. Asp. 2000;164:27–38. doi: 10.1016/S0927-7757(99)00389-1. [DOI] [Google Scholar]
- 4.Ahsan F., Arnold J., Meezan E., Pillion D. Sucrose cocoate, a component of cosmetic preparations, enhances nasal and ocular peptide absorption. Int. J. Pharm. 2003;251:195–203. doi: 10.1016/s0378-5173(02)00597-5. [DOI] [PubMed] [Google Scholar]
- 5.Cázares-Delgadillo J., Naik A., Kalia Y.N., Quintanar-Guerrero D., Ganem-Quintanar A. Skin permeation enhancement by sucrose esters: A pH-dependent phenomenon. Int. J. Pharm. 2005;297:204–212. doi: 10.1016/j.ijpharm.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Csóka G., Marton S., Zelko R., Otomo N., Antal I. Application of sucrose fatty acid esters in transdermal therapeutic systems. Eur. J. Pharm. Biopharm. 2007;65:233–237. doi: 10.1016/j.ejpb.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Szűts A., Pallagi E., Regdon G., Jr., Aigner Z., Szabó-Révész P. Study of thermal behaviour of sugar esters. Int. J. Pharm. 2007;336:199–207. doi: 10.1016/j.ijpharm.2006.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Chortyk O.T., Pomonis J.G., Johnson A.W. Syntheses and characterizations of insecticidal sucrose esters. J. Agric. Food Chem. 1996;44:1551–1557. doi: 10.1021/jf950615t. [DOI] [Google Scholar]
- 9.Pouillart P., Cerutti I., Ronco G., Villa P., Chany C. Butyric monosaccharide ester-induced cell differentiation and anti-tumor activity in mice. Importance of their prolonged biological effect for clinical applications in cancer therapy. Int. J. Cancer. 1991;49:89–95. doi: 10.1002/ijc.2910490117. [DOI] [PubMed] [Google Scholar]
- 10.Calabresse C., Venturini L., Ronco G., Villa P., Chomienne C., Belpomme D. Butyric acid and its monosaccharide ester induce apoptosis in the HL-60 cell line. Biochem. Biophys. Res. Commun. 1993;195:31–38. doi: 10.1006/bbrc.1993.2005. [DOI] [PubMed] [Google Scholar]
- 11.Pouillarta P., Douilleta O., Scappinib B., Gozzinib A., Santinib V., Grossi A., Pagliai G., Strippoli P., Rigacci L., Ronco G., et al. Regioselective synthesis and biological profiling of butyric and phenylalkylcarboxylic esters derivated from D-mannose and xylitol: influence of alkyl chain length on acute toxicity. Eur. J. Pharm. Sci. 1998;7:93–106. doi: 10.1016/s0928-0987(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 12.Santini V., Gozzini A., Scappini B., Caporale R., Zoccolante A., Rigacci L., Gelardi E., Grossi A., Alterini R., Ferrini P.R. Induction of apoptosis by monosaccharide butyrate stable derivatives in chronic lymphocytic leukemia cells. Haematologica. 1999;84:897–904. [PubMed] [Google Scholar]
- 13.Wang C.J., Wang Y.X., Song J.Y., Zhao J. Study on the synthesis and biological activity of diacid solanesyl galactosyl diesters (in Chinese) Chin. Chem. Bull. 2004;67:1–4. [Google Scholar]
- 14.Ferrer M., Soliveri J., Plou F.J., López-Cortés N., Reyes-Duarte D., Christensen M., Copa-Patiño J.L., Ballesteros A. Synthesis of sugar esters in solvent mixtures by lipases from Thermomyces lanuginosus and Candida antarctica B, and their antimicrobial properties. Enzym. Microb. Technol. 2005;36:391–398. doi: 10.1016/j.enzmictec.2004.02.009. [DOI] [Google Scholar]
- 15.Zhou R.J., Huang Y.X., Zeng X., Huang M., Zhang Q., Lin P.X., Zhong G.J., Qi K.Q. Synthesis and antimicrobial activity of Sucrose Methyl Fumarate (in Chinese) Food Sci. Technol. 2007;32:193–197. [Google Scholar]
- 16.Habulin M., Šabeder S., Knez Ž. Enzymatic synthesis of sugar fatty acid esters in organic solvent and in supercritical carbon dioxide and their antimicrobial activity. J. Supercrit. Fluids. 2008;45:338–345. doi: 10.1016/j.supflu.2008.01.002. [DOI] [Google Scholar]
- 17.Huang D., Zhong K.G., Zhu W.F., Gao W.D. Ultrasonic synthesis, characteristic and application of sucrose octanoate. Chin. J. Org. Chem. 2009;29:1951–1955. [Google Scholar]
- 18.Řiháková Z., Plocková M., Filip V., Šmidrkal J. Antifungal activity of lauric acid derivatives against Aspergillus niger. Eur. Food Res. Technol. 2001;213:488–490. doi: 10.1007/s002170100416. [DOI] [Google Scholar]
- 19.Yu H., Ning Z.X. Synthesis and antibacterial activity of α-furan acrylate (in Chinese) Sci. Technol. Food Ind. 2005;26:145–147. [Google Scholar]
- 20.Zhang Q., Teng J.J., Qiao Y.H., Huang Z.X., Chen X.D. Study on synthesis of sucrose α-furylacrylate and its inhibiting activity (in Chinese) Food Sci. Technol. 2009;34:222–224. [Google Scholar]
- 21.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 22.Soković M.D., Vukojević J., Marin P.D., Brkić D.D., Vajs V., van Griensven L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules. 2009;14:238–249. doi: 10.3390/molecules14010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soković M., Glamočlija J., Marin P.D., Brkić D., van Griensven L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal Herbs using an In Vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopalan S., Raman P.V.A. Organic Syntheses. Vol. 3. John Wiley & Sons. Inc.; New York, NY, USA: 1963. Furylacrylic Acid; pp. 425–427. [Google Scholar]
- 25.Da Fde Silva C., de Souza M.C.B.V., Frugulhetti I.I.P., Castro H.C., de O. Souza S.L., de Souza T.M.L., Rodrigues D.Q., Souza A.M.T., Abreu P.A., Passamani F., et al. Synthesis, HIV-RT inhibitory activity and SAR of 1-benzyl-1H-1,2,3-triazole derivatives of carbohydrates. Eur. J. Med. Chem. 2009;44:373–383. doi: 10.1016/j.ejmech.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 26.Li Z.A., Liang X.M., Wu F., Wan B.S. A convenient resolution method for 1,1'-bi-2-naphthol and 4,4'-dibromo-1,1'-spirobiindane-7,7'-diol with menthyl chloroformate in the presence of TBAB. Tetrahedron Asymmetry. 2004;15:665–669. [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. Document M100-S12. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 2002. [Google Scholar]
- 28.National Committee for Clinical Laboratory Standards. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved standard M27-A. National Committee for Clinical Laboratory Standards; Wayne, PA, USA: 1997. [Google Scholar]