Abstract
The essential oils obtained from leaves of Piper duckei and Piper demeraranum by hydrodistillation were analyzed by gas chromatography-mass spectrometry. The main constituents found in P. demeraranum oil were limonene (19.3%) and β-elemene (33.1%) and in P. duckei oil the major components found were germacrene D (14.7%) and trans-caryophyllene (27.1%). P. demeraranum and P. duckei oils exhibited biological activity, with IC50 values between 15 to 76 μg mL−1 against two Leishmania species, P. duckei oil being the most active. The cytotoxicity of the essential oils on mice peritoneal macrophage cells was insignificant, compared with the toxicity of pentamidine. The main mono- and sesquiterpene, limonene (IC50 = 278 μM) and caryophyllene (IC50 = 96 μM), were tested against the strains of Leishmania amazonensis, and the IC50 values of these compounds were lower than those found for the essential oils of the Piper species. The HET-CAM test was used to evaluate the irritation potential of these oils as topical products, showing that these oils can be used as auxiliary medication in cases of cutaneous leishmaniasis, with less side effects and lower costs.
Keywords: Piper duckei, Piper demeraranum, essential oil, Leishmaniasis, HET-CAM
1. Introduction
Leishmaniasis, one of the parasitic diseases spread worldwide, with about 12 million infected people, presents an increasing number of new cases [1]. In Brazil, studies report the occurrence of about 20.000 new cases of the illness annually [2]. Leishmaniasis is caused by parasites of the genus Leishmania, after transmission by phlebotominae (sandflies). The chemotherapeutic agents used for the treatment of leishmaniasis such as sodium stibogluconate, N-methylglucamine antimonate, pentamidine and amphotericin B are not orally active and require a long-term parenteral administration. These agents also present severe side effects such as cardio and renal toxicities and are expensive [3]. Additionally, parasites of the genus Leishmania are increasingly resistant to available antileishmanial agents, and so the identification of new compounds that could be active against these parasites is an urgent priority [4]. The majority of this research is based on the use of natural products.
The discovery of new drugs from natural products against various diseases, including parasitic diseases such as malaria and leishmaniasis [5,6,7] has gained new impetus in recent years, especially if we observe that a considerable amount of the drugs used today are derived from natural products [8,9]. In this respect, ethnopharmacology has an important role in the identification of medicinal plants with pharmacological potential [9,10]. Medicinal plants and their extracts have been used for many centuries to treat different diseases and their essential oils and/or active constituents can be a source of alternative or adjunct phytomedicines for the treatment of parasites. Many treatments, including topical therapy, have been suggested for this disease and in this respect, plant extracts may be effective [11].
The Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) assay has been used to assess the irritation potential of substances [12] and plant extracts, and has proven useful in screening natural products [13]. These in vivo CAM screening tests were comparable to tests on human skin, and a similar level of efficacy was obtained [13].
Piper species are widely used in folk medicine in Latin America and the West Indies [14], to heal wounds, reduce swelling and skin irritations, and treat the symptoms of cutaneous leishmaniasis [15]. Several studies emphasize the importance of Piper species in the treatment of this disease [16,17,18,19,20,21]. In fact, various classes of antiparasitic active compounds have been identified in Piper species, such as chalcones and dihydrochalcones [22,23], benzoic acid derivatives [17] and neolignans [21].
The chemical composition of essential oils of Piper is mainly comprised of phenylpropanoids such as safrole, dillapiol and myristicin, or terpenes such as limonene, β-caryophyllene, spathulenol, (E)-nerolidol, α-bicyclogermacrene and cadinol [19,24,25]. Insecticidal, fungicidal, bactericidal, larvicidal and molluscicidal properties are attributed to these species [25]. Also, Guerrini and collaborators [19] carried out extensive pharmacological evaluation of essential oils obtained from P. aduncum and P. obliquum, with interesting results.
In this context, encouraged and inspired by reports that show the activity of Piper species against leishmaniasis, and by a few reports on P. demeraranum and P. duckei [26,27,28], we decided to carry out a study on these two species with frequent distribution in the Amazonas state of Brazilian Amazonia.
This present study investigated the chemical composition, antileishmanial and cytotoxic activities and irritation potential, through in vivo CAM assays, of essential oils obtained from leaves of P. demeraranum and P. duckei. Additionally, two main constituents, (−) limonene and caryophyllene, were tested against strains of Leishmania amazonensis.
2. Results and Discussion
Chemical Study
The yields of the essential oils obtained from the leaves of P. demeraranum and P. duckei were 0.6 and 0.5%, respectively. The optical rotations of the oils were P. demeraranum
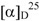 = −1.30° (CHCl3, c 1.75) and P. duckei
= −1.30° (CHCl3, c 1.75) and P. duckei 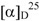 = +1.38° (CHCl3, c 1.30). Table 1 lists the constituents of the essential oils of P. demeraranum and P. duckei analyzed by GC-MS. Although aromatic compounds are common constituents in the essential oils produced by the Piperaceae species, none were detected in the oils analyzed. In total, 25 different compounds were identified and the results showed that the essential oils of these species are rich in sesquiterpenes.
= +1.38° (CHCl3, c 1.30). Table 1 lists the constituents of the essential oils of P. demeraranum and P. duckei analyzed by GC-MS. Although aromatic compounds are common constituents in the essential oils produced by the Piperaceae species, none were detected in the oils analyzed. In total, 25 different compounds were identified and the results showed that the essential oils of these species are rich in sesquiterpenes.
Table 1.
Chemical composition of the leaf essential oils of P. demeraranum and P. duckei.
| Compounds | Oil Composition (%) | ||
|---|---|---|---|
| RI | P. demeraranum | P. duckei | |
| Monoterpenes | |||
| α−Pinene | 932 | 3.9 | 0.7 |
| β-Pinene | 974 | 6.7 | 0.4 |
| (-)-Limonene | 1024 | 19.3 | - |
| 1,8 – Cineole | 1026 | - | 5.8 |
| Sesquiterpenes | |||
| δ-Elemene | 1335 | 1.0 | 0.1 |
| α-Copaene | 1374 | 0.5 | 1.6 |
| β-Elemene | 1389 | 33.1 | - |
| trans-Caryophyllene | 1417 | 6.0 | 27.1 |
| β-Copaene | 1430 | - | 0.5 |
| α-Humulene | 1452 | - | 2.5 |
| ( Z)-Muurola-4-(14),5-diene | 1465 | - | 1.1 |
| Germacrene-D | 1484 | 5.2 | 14.7 |
| β-Selinene | 1489 | 5.0 | - |
| Bicyclogermacrene | 1497 | 8.8 | 5.2 |
| α-Muurolene | 1500 | - | 1.4 |
| ( E,E)-α-Farnesene | 1505 | 1.0 | - |
| γ-Cadinene | 1513 | - | 1.8 |
| δ-Cadinene | 1522 | 1.3 | 3.9 |
| α-Calacorene | 1544 | - | 0.3 |
| Germacrene B | 1559 | 1.1 | - |
| ( E)-Nerolidol | 1561 | - | 0.4 |
| Guaiol | 1600 | - | 1.1 |
| 1- epi-Cubenol | 1627 | - | 0.6 |
| γ-Eudesmol | 1630 | - | 17.9 |
| α-Muurolol | 1644 | - | 3.0 |
| Terpenoids classes | |||
| Monoterpene hydrocarbons | 29.9 | 1.1 | |
| Oxygenated monoterpenes | - | 5.8 | |
| Sesquiterpene hydrocarbons | 63.0 | 60.2 | |
| Oxygenated sesquiterpenes | - | 23.0 | |
| Identified Components (%) | 92.9 | 90.1 | |
Regarding the content of monoterpenes when comparing the two species, P. demeraranum presented a higher concentration (29.9%), with limonene (19.3%) being its major compound. The main sesquiterpene of the essential oil of P. demeraranum was β-elemene (33.1%), while that of P. duckei was trans-caryophyllene (27.1%). Great amount of oxygenated sesquiterpenes was found only in the oil of P. duckei (23.0%). Santos et al. [26] collected P. duckei in another area of the State of Amazonas, and reported a different major constituent for essential oil and no monoterpenes, presumably due to the extraction process used. A total of 6.9% of monoterpenes were identified by us in the essential oil of P. duckei.
Also, 19 mono- and sesquiterpenes were detected, representing 29.9% and 63.0% of the total essential oil of P. demeraranum. This terpene class of P. demeraranum essential oil from the State of Amazonas was therefore different when compared with the species collected in the State of Pará by Andrade et al. [28]. In addition, there are other factors that influence the difference between these results, which are not part of this study. Here, the sesquiterpene β-elemene (33.1%) was the major constituent, followed by the monoterpene limonene (19.3%), the major constituent reported by Andrade et al. [28] for Pará oil.
Both Piper essential oils inhibited the growth of promastigote forms in the two species of Leishmania tested, with IC50 of between 15 and 76 μg mL−1. The essential oils of P. duckei (IC50 = 15.2 μg mL−1) and P. demeraranum (IC50 = 22.7 μg mL−1) indicated greater activity against L. guyanensis promastigotes and weakened inhibition of L. amazonenis (Table 2). These oils inhibited the growth of amastigote forms of L. amazonensis, again showing more activity than the P. duckei oil (IC50 = 42.4 μg mL−1). The non-parasitized peritoneal macrophages were preserved at the concentrations of the essential oils, which were toxic to the parasite. Even when used at concentrations seven times higher, both essential oils were less toxic than pentamidine, proving to be highly relevant for future use.
Table 2.
Leishmanicidal and Cytotoxicity Activities of Piper demeraranum and P. duckei Essential Oils.
| Samples | L. amazonensis | L. guyanensis | Mice (Balb/c) macrophages | ||
|---|---|---|---|---|---|
| Promastigotes | Amastigotes | Promastigotes | |||
| IC50 ± SD | IC50 ± SD (µg/mL) | IC50 ± SD (µg/mL) | Cytotoxicity / (%) | ||
| P. demeraranum | 86.0 ± 2.4 µg/mL | 78 ± 1.0 | 22.7 ± 1.1 | IC50 7 × IC50 | 14.422.3 |
| P. duckei | 46.0 ± 1.3 µg/mL | 42.4 ± 0.8 | 15.2 ± 0.9 | IC50 7 × IC50 | 16.824.4 |
| Limonene | 278 ± 12 µM | - | - | - | - |
| trans -Caryophyllene | 96 ± 19 µM | - | - | - | - |
| Pentamidine | 4.6 ± 0.4 µg/mL | - | 0.8 ± 0.2 | - | 26.2 |
Various studies have demonstrated significant activity of mono- and sesquiterpenes against Leishmania species [5,29], but few studies have been conducted using essential oil from Piper species [20]. The antileishmanial activity of P. demeraranum oil may be associated with the main monoterpene limonene, which has been linked to several biological activities [30,31], including antimalarial [32] and antileishmanial activity [33]. Some sesquiterpenoids have been described as promising substances for the treatment of leishmaniasis. In fact, several oxygenated sesquiterpenes have evidenced significant activity against Leishmania species [5,7]. These reports are consistent with the activity found for these substances in this work.
The main mono- and sesquiterpene of the oils, (−) limonene and trans-caryophyllene, respectively, were evaluated against promastigote forms of L. amazonensis and showed IC50 value of 278 μM and 96 μM, respectively, both being more active than their corresponding essential oils. Among these activities, the antileishmanial activity of trans-caryophyllene was at least two times more potent than limonene. The inhibitory effect of limonene against L. amazonensis was similar to that described by Arruda and collaborators [33], but this is the first description of caryophyllene with this Leishmania species.
It is worth mentioning that during the experiments, it was observed that the purity of caryophyllene is an important factor for the activity against L. amazonensis. The oxidation of caryophyllene into its corresponding oxides affects the results and depending on this oxidation level, the activity cannot be observed (data not shown).
At this point, it was found that the oils under study were not toxic; that they showed antileishmanial activity; and that they can be obtained from renewable parts of the plant. Therefore, based on the popular use of Piper species for the treatment of skin wounds, further assays were conducted to evaluate the potential of these oils for use as topical medicine. The HET-CAM assay was used to show the irritation potential of these essential oils because it is rapid and inexpensive. The oils were applied without complications. The data from this assay were quite promising, featuring oils with slight and moderate skin irritations at a dose calculated to be about 10 times the IC50 of L. amazonensis. The results of the irritation induced by essential oils are described in Table 3. Water was used as the negative reference substance and a second control, 0.1% DMSO + water (used to dissolve the oils), was used and did not provoke any reaction. A positive control such as sodium dodecyl sulphate (SDS) 1% was also tested. The concentration used for both oils did not cause bleeding or clotting of the membrane. The essential oils of P. duckei and P. demeraranum had an irritation score (IS) of 4.9 and 5.1, indicating a slightly irritant and moderately irritant effect, respectively, as described by Luepke [12] at concentrations above those observed for parasite growth inhibition.
Table 3.
Assessment of irritation potential of Piper oils tested in the CAM.
| Samples | Congestion | Haemorrhage | Coagulation | Irritation score | Irritation Assessment |
|---|---|---|---|---|---|
| Control (-) | 0 | 0 | 0 | 0 | Non-irritant |
| 2sd Control | 0 | 0 | 0 | 0 | Non-irritant |
| Control (+) | 5 | 5.7 | 4.7 | 15.4 | Strong |
| PDU (500 µg) | 1.8 | 0 | 0 | 4.9 | Slight |
| PDE (900 µg) | 1.7 | 0 | 0 | 5.1 | Moderate |
Control (+): sodium lauryl sulfate 1%; Control (−): water; 2sd Control: water + DMSO 0.1%; PDU: P. duckei; PDE: P. demeraranum.
These results are promising for in vivo tests, especially if one considers the low density and high lipophilicity of these oils and their components, which have rapid transdermal penetration, facilitating their action [34]. Another important point is the presence of a terminal methylene group in the structure of the substances tested, described as an important site that has harmful effects on the microorganisms [35]. Nevertheless, further studies are required to explain the complete mechanisms of these interactions, particularly in the specific case of leishmaniasis.
The discovery of topical medications for the treatment of leishmaniasis has increased, gaining support from various agencies, such the World Health Organization (WHO). These results are relevant, since the development of new phytotherapeutic agents containing essential oils, or their active compounds, as well as their application in cutaneous leishmaniasis, can have a positive impact on this worldwide neglected disease, with less side effects and lower costs.
3. Experimental
3.1. Plant Material
The specimens of P. duckei (PDU) and P. demeraranum (PDE) were collected in the Adolpho Ducke reserve, Km 26 Manaus – Itacoatiara highway, in the State of Amazonas, Brazil. Voucher specimens were deposited at the Herbarium of the Institute of Biological Sciences (UFAM) under the numbers 188224 (PDU) and 188187 (PDE).
3.2. Extraction of Essential Oil
The leaves of P. duckei and P. demeraranum (600 g) were dried at room temperature, ground, and submitted to hydrodistillation (4 h) using a modified Clevenger-type apparatus. After the end of each distillation, the oils were collected, transferred to glass flasks, and stored at a temperature of −4 °C. The yields were calculated based on the weight of the plant material used. (−) Limonene (96%) and trans-caryophyllene (98.5%) were supplied by Sigma –Aldrich Chemical Co and Fluka, respectively.
3.3. Essential Oil Analysis
The oil composition was performed by comparison of their retention indices and mass spectra with those reported in the literature [36] or stored in the Wiley data system library. The retention indices were calculated for all volatile constituents using n-alkane homologous series. GC-MS analyses were performed using an Agilent 6890N gas chromatograph interfaced with an Agilent 5973N Mass Selective Detector (ionization voltage 70 eV), fitted with a DB-5MS column (30 m × 0.25 mm, film thickness 0.25 μm), using helium as the carrier gas at 1.0 mL min−1. The oven temperature was programmed from 60 °C to 290 °C at a rate of 3 °C min−1, and hydrogen was used as carrier gas (1.0 mL min−1). Injector and detector temperatures were 230 °C and 280 °C, respectively. Optical rotations were measured in 15 mm cell at 25° C at 589 nm (sodium D-Line) with a digital polarimeter JASCO DIP-370.
3.4. Anti-leishmanial Assay
Promastigotes of Leishmania amazonensis (MHOM/BR/77LTB0016 strain) and L. guyanensis (MHOM/BR/95/IOCL-2092/IM4216 strain) isolated from patients with cutaneous leishmaniasis, the latter in Manaus, were routinely cultured at 26ºC in Schneider’s medium [37] supplemented with 10% fetal calf serum (FCS), pH 7.2.
Parasites (promastigotes) were harvested from the medium on day three of culture, resuspended in fresh medium, counted in a Neubauer chamber, and adjusted to a concentration of 4 × 106 parasites mL−1, for the drug assay. The essential oils to be tested were dissolved in DMSO (the highest concentration was 1.4%, which was not hazardous to the parasites) and added to parasite suspensions at a final concentration of between 0.156 and 320 μg mL−1. The standards of limonene and caryophyllene were dissolved in DMSO at concentrations of 60, 30, 15 and 7.5 μg/mL. After 24 h of incubation, the parasites were counted and compared with the controls containing DMSO and parasites alone. All the tests were done in triplicate and Pentamidine isethionate was used as the reference drug. The concentration causing 50% inhibition (IC50) was obtained from the drug concentration—response curve and the results were expressed as the mean ± standard deviation determined from three independent experiments [38,39].
Approximately 4 × 106 BALB/c mice peritoneal macrophages were placed in a 24-well plate in complete RPMI medium (Sigma Cell Culture, St. Louis, MO, USA). L. amazonensis promastigote forms in a stationary phase of culture growth were added to the macrophage at a ratio of 10 parasites to 1 macrophage. The culture was incubated at 5% CO2 at 35 °C and after 24 h, the plate was washed with phosphate buffer solution (PBS) at 25 °C to remove non-internalized promastigotes. A new culture medium at 25 °C was added and the essential oil was added to the infected macrophages at concentrations of 60, 30, 15 and 7.5 μg mL−1. After 24 h, the infected macrophages were fixed and stained with Giemsa to verify the amastigote counts within the macrophages [40].
3.5. Cytotoxicity Assay
In order to evaluate the toxicity of the sample for the host cell, mice peritoneal macrophages were isolated in RPMI 1640 medium, containing 200 UI mL−1 penicillin, 200 μg mL−1 of streptomycin, 1 mM sodium piruvate, 1 mM of L-glutamine and 1M HEPES buffer (Sigma Cell Culture, St. Louis, MO, USA). Cells were counted in Neubauer’s chamber using erythrosine B as vital dye (Sigma Cell Culture) and adjusted to a concentration of 4 × 106 cells mL−1. Cells were then cultured in a 96 wells culture plate (Falcon, NJ, USA) at 37 °C and in an atmosphere of 5% CO2. The sample was added to the medium in a concentration equivalent to IC50 and 7× IC50 of the in vitro activity assay from L. guyanensis. The sample and pentamidine isethionate (reference drug) were added to the culture and after 24 h, the viability of treated cells was compared to that of the control without drugs, through the MTT methodology [41].
3.6. Statistical Analysis
Statistical significance (p < 0.05) was evaluated by the ANOVA test followed by Student-Newman-Keuls, by Kruskall-Wallis or Mann-Whitney tests software SPSS for Windows.
3.7. HET-CAM Test (Hen’s Egg Test—Chorioallantoic Membrane)
The method of Luepke [12] was used. The test samples were dissolved and dropped onto the chorioallantoic membrane at a volume of 0.2 mL, followed by the controls. In every case, a series of five eggs was used. After the application of the sample, the CAM, the blood vessels, including the capillary system, and the albumen were examined. The ensuing irritation reaction was observed over 5 min, with the time (sec) to first appearance of each hemorrhage, lysis and coagulation recorded as in irritancy assays (congestion, haemorrhage, coagulation). According to Lupke [12], the results can be expressed in the following categories: non-irritant (0.0–0.9), slight irritant (1.0–4.9), moderate irritant (5.0–8.9) and strong irritant (9.0–21.0).
4. Conclusions
In general, the acquisition of essential oils from plants is an uncomplicated and low cost procedure. Among the oils studied in this article, there is an obvious difference in the chemical constituents of them. The essential oil of Piper duckei as well as its main component, trans-caryophyllene, were the most active against Leishmania species. Further significant points, an insignificant toxicity and a low level of irritability observed for the oils analyzed, confirming possible utilization of these oils or compounds as auxiliary medication in cases of cutaneous leishmaniasis.
Acknowledgements
The authors thank FAPEAM (PPP and PIPT), PAPES V/CNPq Project number 403481/2008-2 for the research grants and June Justa for the English revision of the manuscript. DFM thanks the CNPq fellowship and E. N. Alves for assistance with the HET-CAM assays.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1. World Health Organization. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniasis; Technical Report Series 949; WHO: Geneva, Switzerland, 2010; p. 12. [Google Scholar]
- 2.Ministério da Saúde 2011. [(accessed on 5 January 2012)]. Available online: http://portal.saude.gov.br/portal/saude/profissional/area.cfm?id_area=1560/
- 3.Akendengue B., Ngou-Milama E., Laurens A., Hocquemiller R. Recent advances in the fight against leishmaniasis with natural products. Parasite. 1999;6:3–8. doi: 10.1051/parasite/1999061003. [DOI] [PubMed] [Google Scholar]
- 4.Natera S., Machuca C., Padrón-Nieves M., Romero A., Díaz E., Ponte-Sucre A. Leishmania spp.: Proficiency of drug-resistant parasites. Int. J. Antimicrob. Agents. 2007;29:637–642. doi: 10.1016/j.ijantimicag.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Chan-Bacab M.J., Pena-Rodriguez L.M. Plant natural products with leishmanicidal activity. Nat. Prod. Rep. 2001;18:674–688. doi: 10.1039/b100455g. [DOI] [PubMed] [Google Scholar]
- 6.Pink R., Hudson A., Mouriès Marie-Annick, Bendig M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005;4:727–740. doi: 10.1038/nrd1824. [DOI] [PubMed] [Google Scholar]
- 7.Rocha L.G., Almeida J.R.G.S., Macêdo R.O., Barbosa-Filho J.M. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–535. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B.M., Ribnicky D.M., Lipsky P.E., Raskin I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 2007;3:360–366. doi: 10.1038/nchembio0707-360. [DOI] [PubMed] [Google Scholar]
- 10.Leonti M. The future is written: Impact of scripts on the cognition, selection, knowledge and transmission of medicinal plant use and its implications for ethnobotany and ethnopharmacology. J. Ethnopharmacol. 2011;134:542–555. doi: 10.1016/j.jep.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Jaffary F., Nilforoushzadeh M.A., Moradi S., Derakhshan R., Ansari N. The Efficacy of Topical Treatment of Concentrated Boiled Extract and Hydroalcoholic Extract of Cassia fistula in Comparison to the Intralesional Injection of Meglumine Antimoniate in the Treatment of Acute Cutaneous Leishmaniasis. J. Skin Leish. 2001;1:23–27. [Google Scholar]
- 12.Luepke N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985;23:287–291. doi: 10.1016/0278-6915(85)90030-4. [DOI] [PubMed] [Google Scholar]
- 13.Wilson T.D., Steck W.F. A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem. Toxicol. 2000;38:867–872. doi: 10.1016/S0278-6915(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 14.Parmar V.S., Jain S.C., Bisht K.S., Jain R., Taneja P., Jha A., Tyagi O.D., Prasad A.K., Wengel J., Olsen C.E., Boll P.M. Phytochemistry of the genus Piper. Phytochemistry. 1997;46:597–673. [Google Scholar]
- 15.Estevez Y., Castillo D., Pisango M.T., Arevalo J., Rojas R., Alban J., Deharo E., Bourdy G., Sauvain M. Evaluation of the leishmanicidal activity of plants used by Peruvian Chayahuita ethnic group. J. Ethnopharmacol. 2007;114:254–259. doi: 10.1016/j.jep.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Braga F.G., Bouzada M.L.M., Fabri R., Matos M.O., Moreira F.O., Scio E., Coimbra E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007;111:396–402. doi: 10.1016/j.jep.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Flores N., Jiménez I.A., Giménez A., Ruiz G., Gutiérrez D., Bourdy G., Bazzocchi I.L. Benzoic acid derivatives from Piper species and their antiparasitic activity. J. Nat. Prod. 2008;71:1538–1543. doi: 10.1021/np800104p. [DOI] [PubMed] [Google Scholar]
- 18.Sarkar A., Sen R., Saha P., Ganguly S., Mandal G., Chatterjee M. An ethanolic extract of leaves of Piper betle (Paan) Linn mediates its antileishmanial activity via apoptosis. Parasitol. Res. 2008;102:1249–1255. doi: 10.1007/s00436-008-0902-y. [DOI] [PubMed] [Google Scholar]
- 19.Guerrini A., Sacchetti G., Rossi D., Paganetto G., Muzzoli M., Andreotti E., Tognolini M., Maldonado M.E., Bruni R. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharm. 2009;27:39–48. doi: 10.1016/j.etap.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Monzote L., García M., Montalvo A.M., Scull R., Miranda M. Chemistry, cytotoxicity and antileishmanial activity of the essential oil from Piper auritum. Mem. Inst. Oswaldo Cruz. 2010;105:168–173. doi: 10.1590/S0074-02762010000200010. [DOI] [PubMed] [Google Scholar]
- 21.Vendrametto M.C., dos Santos A.O., Nakamura C.V., Filho B.P.D., Cortez D.A.G., Ueda-Nakamura T. Evaluation of antileishmanial activity of eupomatenoid-5, a compound isolated from leaves of Piper regnellii var. pallescens. pallescens. Parasitol. Int. 2010;59:154–158. doi: 10.1016/j.parint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Caio T.S.E., Lima M.D., Kaplan M.A.C., Nazareth M.M., Rossi-Bergmann B. Selective effect of 2’,6’-dihydroxy-4’methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999;43:1234–1241. doi: 10.1128/aac.43.5.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flores N., Cabrera G., Jiménez I.A., Piñero J., Giménez A., Bourdy G., Cortés-Selva F., Bazzocchi I.L. Leishmanicidal constituents from the leaves of Piper rusbyi. Planta Med. 2007;73:206–211. doi: 10.1055/s-2007-967123. [DOI] [PubMed] [Google Scholar]
- 24.Santos P.R.D., Moreira D.L., Guimarães E.F., Kaplan M.A.C. Essential oil analysis of 10 Piperaceae species from the Brazilian Atlantic forest. Phytochemistry. 2001;58:547–551. doi: 10.1016/s0031-9422(01)00290-4. [DOI] [PubMed] [Google Scholar]
- 25.Maia J.G.S., Andrade E.H.A. Database of the Amazon aromatic plants and their essential oils. Quim. Nova. 2009;32:595–622. doi: 10.1590/S0100-40422009000300006. [DOI] [Google Scholar]
- 26.Santos A.S., Andrade E.H.A., Zoghbi M.G.B., Luz A.I.R., Maia J.G.S. Sesquiterpenes of Amazonian Piper species. Acta Amazon. 1998;28:127–130. [Google Scholar]
- 27.Maxwell A., Rampersa D. A new amide from Piper demeraranum. J. Nat. Prod. 1989;52:891–892. doi: 10.1021/np50064a043. [DOI] [Google Scholar]
- 28.Andrade E.H.A., Guimarães E.F., Maia J.G.S. Essential Oil Composition of Piper demeraranum (Mix.) C. DC. J. Essent. Oil Bear. Pl. 2006;9:47–52. [Google Scholar]
- 29.Farnsworth N.R., Bingel A.S. Problems and prospects of discovering new drugs from higher plants by pharmacological screening. In: Wagner H., Wolff P., editors. New Natural Products and Plant Drugs with Pharmacological, Biological or Therapeutical Activity. Springer-Verlag; Berlin, Germany: 1977. pp. 1–22. [Google Scholar]
- 30.Uedo N., Tatsuta M., Iishia H., Baba M., Sakai N., Yano H., Otani T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999;137:131–136. doi: 10.1016/S0304-3835(98)00340-1. [DOI] [PubMed] [Google Scholar]
- 31.Singh P., Shukla R., Prakash B., Kumar A., Singh S., Mishra P.K., Dubey N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem. Toxicol. 2010;48:1734–1740. doi: 10.1016/j.fct.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Goulart H.R., Kimura E.A., Peres V.J., Couto A.S., Duarte F.A.A., Katzin A.M. Terpenes Arrest Parasite Development and Inhibit Biosynthesis of Isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004;48:2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arruda D.C., Miguel D.C., Yokoyama-Yasunaka J.K.U., Katzin A.M., Uliana S.R.B. Inhibitory activity of limonene against Leishmania parasites in vitro and in vivo. Biomed. Pharmacother. 2009;63:643–649. doi: 10.1016/j.biopha.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Jean-Paul A., Fyfe L., Smith H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Nibret E., Wink M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine. 2010;17:911–920. doi: 10.1016/j.phymed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Adams R.P. Identification of Essential oil Components by Gas chromatography/Quadrupole Mass Spectroscopy. Allured; Carol Stream, IL, USA: 2001. p. 455. [Google Scholar]
- 37.Hendricks L.D., Wood D.E., Hajduk M.E. Haemoflagellates: Commercially available liquid media for rapid cultivation. Parasitology. 1978;76:309–316. doi: 10.1017/S0031182000048186. [DOI] [PubMed] [Google Scholar]
- 38.Silva J.R.A., do Carmo D.F.M., Reis E.M., Gérzia Machado M.C., Leon L.L., Silva B.O., Ferreira J.L.P., Amaral A.C.F. Chemical and Biological Evaluation of Essential Oils with Economic Value from Lauraceae Species. J. Braz. Chem. Soc. 2009;20:1071–1076. doi: 10.1590/S0103-50532009000600011. [DOI] [Google Scholar]
- 39.Costa E.V., Pinheiro M.L., Xavier C., Silva J., Amaral A.C.F., Souza A., Campos F., Ferreira A., Machado G.M.C., Leon L.L. A pyrimidine-beta-carboline and others alkaloids from Annona foetida with antileishmanial activity. J. Nat. Prod. 2006;69:292–294. doi: 10.1021/np050422s. [DOI] [PubMed] [Google Scholar]
- 40.da Silva E.F., Canto-Cavalheiro M.M., Braz V.R., Cysne-Finkelstein L., Leon L.L., Echevarria A. Synthesis, and biological evaluation of new 1,3,4-thiadiazolium-2-phenylamine derivatives against Leishmania amazonensis promastigotes and amastigotes. Eur. J. Med. Chem. 2002;37:979–984. doi: 10.1016/S0223-5234(02)01401-0. [DOI] [PubMed] [Google Scholar]
- 41.Mosmann T. Rapid colorimetric assay for cellular growth and survival. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]


