Figure 3.
EMC Is Required for Accurate TMD1 Topogenesis of β1AR
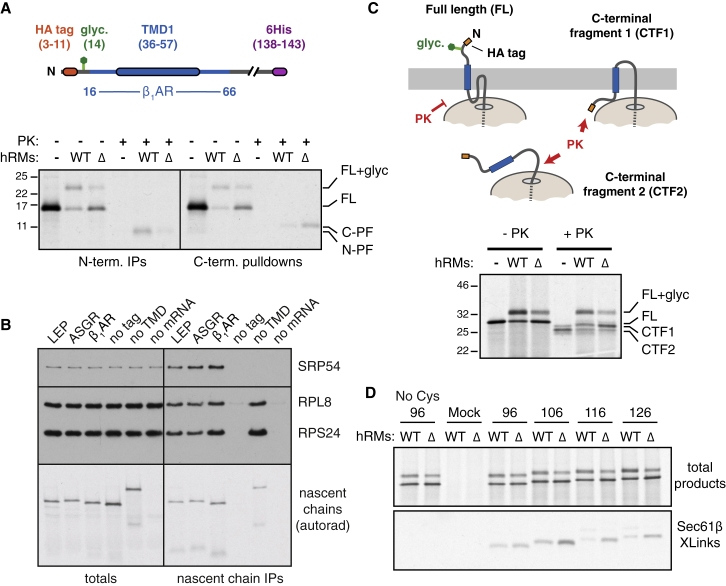
(A) 35S-methionine labeled β1AR-TMD1 (shown in the diagram) was translated in the absence or presence of WT or ΔEMC6 (Δ) hRMs, subjected to PK digestion as indicated, and the products recovered by either immunoprecipitation via the N-terminal HA tag (N-term. IPs) or pull-downs via the C-terminal His6 tag (C-term. pull-downs). The positions of unmodified full-length (FL) product, glycosylated product (+glyc), and N- and C-terminal protease-protected fragments (N-PF and C-PF, respectively) are indicated.
(B) 35S-methionine labeled ribosome-nascent chains (stalled 39 residues downstream of the indicated TMDs) produced in reticulocyte lysate were affinity purified via an N-terminal FLAG epitope tag and analyzed by autoradiography to detect the nascent chains or immunoblotting for ribosomal proteins (RPL8 and RPS24) and SRP54. Controls either lacked an epitope tag, TMD, or mRNA.
(C) 35S-methionine labeled 116-residue nascent chains of β1AR were targeted to WT or ΔEMC6 hRMs and analyzed by the PK protection assay. The diagram indicates which species are glycosylated and PK-resistant versus non-glycosylated and PK-accessible.
(D) 35S-methionine labeled β1AR nascent chains of the indicated lengths were targeted to WT or ΔEMC6 hRMs (top panel), then subjected to sulfhydryl-mediated crosslinking. The crosslinked products were immunoprecipitated using antibodies against Sec61β and shown in the bottom panel. Controls lacking either mRNA (mock) or a cysteine in the nascent chain showed no Sec61β immunoprecipitated products.
See also Figure S4.