Abstract
Cilia are microtubule-based appendages present on almost all vertebrate cell types where they mediate a myriad of cellular processes critical for development and homeostasis. In humans, impaired ciliary function is associated with an ever-expanding repertoire of phenotypically-overlapping yet highly variable genetic disorders, the ciliopathies. Extensive work to elucidate the structure, function, and composition of the cilium is offering hints that the “static” representation of the cilium is a gross oversimplification of a highly dynamic organelle whose functions are choreographed dynamically across cell types, developmental, and homeostatic contexts. Understanding this diversity will require discerning ciliary versus non-ciliary roles for classically-defined “ciliary” proteins; defining ciliary protein-protein interaction networks within and beyond the cilium; and resolving the spatiotemporal diversity of ciliary structure and function. Here, focusing on one evolutionarily conserved ciliary module, the intraflagellar transport system, we explore these ideas and propose potential future studies that will improve our knowledge gaps of the oversimplified cilium and, by extension, inform the reasons that underscore the striking range of clinical pathologies associated with ciliary dysfunction.
Introduction: Celebrating two centuries of progress in the ciliary biology field
Cilia are conserved microtubule-based appendages residing at the apical surface of almost all terminally-differentiated cell types. Based predominantly on the past two decades of research, we now know that cilia function both during embryogenesis and also in differentiated tissues to regulate a multitude of cellular processes[1–3••]. From a historical perspective, cilia are among the oldest observed organelles; they were discovered in the 17th century by Leeuwenhoek and noted for their remarkable motile properties[4]. The appreciation for motile cilia intensified with the realization that multiciliated cells are present in diverse vertebrate tissue types and include cilia lining the respiratory tract, lungs, inner ear, and brain ventricles[5–7]. In 1998, a landmark study uncovered a link between a distinct form of motile cilia in the node and mammalian determination of left-right asymmetry during development[8]. While initial functional studies were focused on motile cilia because of their obvious functions in fluid or cell propulsion, the primary cilium was largely neglected and considered a vestigial structure, despite documentation in the 19th century[9]. Several key findings brought functional relevance to the primary cilium; these include but are not limited to: (1) the causal relationship between primary ciliary dysfunction and the cystic renal pathology in the Oak Ridge Polycystic Kidney mouse (Tg737OPRK)[10,11]; (2) the discovery of a role for primary cilia in Hedgehog (Hh) signal transduction in mice[12]; and (3) the observation that polycystin-1 and polycystin-2, both associated with renal disease, mediate calcium mechanosensation in a primary cilia-dependent manner. [13]. Subsequent in vitro and in vivo work associated the primary cilium to additional morphogenetic pathways including Notch, Wnt, Hippo, mTOR, and PDGFR signaling, redefining the cilium as a cellular antenna critical for development, homeostasis and regenerative processes[14,15•].
Consistent with the near-ubiquitous presence of cilia across tissues, and their critical role in organogenesis and maintenance, it is not surprising that perturbation in cilia structure or function causes a host of human genetic disorders. Primary Ciliary Dyskinesia, characterized by situs inversus, hydrocephalus and chronic airway infections[16], garnered initial attention as a clinical entity caused by impaired motile ciliary beating capacity. In the early 2000s, defects in the primary cilium were implicated as the molecular cause of additional rare human genetic disorders, including isolated renal cystic disease (nephronophthisis; NPHP[17,18]); and Bardet-Biedl syndrome (BBS), a genetically heterogeneous disorder characterized by retinitis pigmentosa, polydactyly, obesity, learning difficulties, and renal anomalies [19]. These studies established a role for cilia in human disease; defined the ciliopathies as a clinical collection of organellar disorders; and led to the identification of a multitude of additional phenotypically-overlapping pathologies[20]. Ciliopathies manifest in a spectrum of hallmark phenotypes with variable penetrance and expressivity[3••,20,21]. Although individually rare, there are ~100 suspected or established cilia-related clinical synopses reported in the Online Mendelian Inheritance in Man database (https://omim.org/) with a collective incidence of ~1:1000, whichis comparable to Down syndrome[22].
A multidisciplinary suite of approaches has been employed to investigate the molecular processes governing ciliogenesis, homeostasis, and pathology. Electron microscopy has helped characterize ciliary ultrastructure[23] and has provided evidence supporting clinical diagnosis for ciliopathies such as Primary Ciliary Dyskinesia[24]. Three main types of cilia have been described based on microtubule arrangements and biological functions: primary (9+0), motile (9+2) and nodal cilia (9+0 microtubule configurations, respectively) and their ultrastructural characteristics have been reviewed extensively elsewhere[25–27], including well-known exceptions to this trichotomy[25].
Given the importance of these organelles in human disease, considerable effort has been directed at cataloguing the protein composition of the cilium[28–39••]. Multiple groups have contributed to the assembly of the ciliary proteome (ciliome), an extensive list of ~1,000 proteins that are found within the ciliary/flagellar axoneme and the underlying basal body/centriole. The core data used to construct the ciliary proteome leveraged the extensive evolutionary conservation of the organelle and integrated mass spectrometry, functional genomics, and comparative genomics data across phyla. Notably, a proteomic study on primary cilia from mouse renal cells showed that 25% of purified proteins were not shared with previously characterized proteomes from motile and specialized sensory cilia, suggesting a subpopulation that is primary cilia-specific[40]. A subset of ciliary proteins has also been grouped into distinct molecular modules identified through biochemical studies performed in vitro. For instance, the BBSome is composed of a subcomplex of eight BBS proteins[41] which has been shown to translocate between the cytoplasm and the transition zone[42] at the ciliary base, and to transport ciliary components within the cilium[43]. Since then, other stable macromolecular complexes have been defined, including the transition zone complex and the NPHP complex, while further evidence has also intimated the existence of a septin pore ring, to name but a few examples[44].
Challenges and Opportunities
Although the characterization of ciliary ultrastructure; protein composition; and cellular functions have been heralded, appropriately, as significant progress, they still represent an overly simplified view of an organelle underpinned by substantial complexity and diversity of composition and function. Intersection of datasets from diverse in vitro models, ranging from renal to retinal epithelial cells, with that of in vivo ciliated models spanning eukaryotic taxa have led to the cartography of a “generic” cilium[39••,40]. From a genetic standpoint, aggregate data suggest that allelism at a single causal locus can account for some clinical diversity. For example, recessive mutations in TTC21B are associated with a phenotypic spectrum ranging from isolated and syndromic NPHP, focal segmental glomerulosclerosis, to the skeletal ciliopathy Jeune Asphyxiating Thoracic Dystrophy[45–47]. To date, nonsense mutations have been observed exclusively in the latter clinical group, while a recurrent p.P209L variant has been associated with isolated renal disease, offering a partial explanation to phenotype diversity. However, there are other ciliopathies for which private missense alleles can cause divergent clinical presentations without a clear genotype-phenotype correlation, such as mutations in IFT172, which can cause isolated retinitis pigmentosa, BBS, or Jeune Asphyxiating Thoracic Dystrophy[48,49]. These differences can likely be explained either by stochastic reasons or by secondary genetic variation affecting either known ciliary proteins, or extra-ciliary processes required for ciliary function[22]. However, genetic information overlaid onto a generic ciliary map is an overly simplistic viewpoint, as evidenced, for example, by a greater susceptibility to retinal pathology in some cases versus skeletal phenotypes in other ciliopathies. A reasonable posit is that some of the clinical variability within ciliopathies can be explained further with: (1) a systems biology approach to understand ciliary and non-ciliary binding proteins; (2) an improved understanding of non-ciliary roles for ciliary proteins; and (3) elucidation of unique spatiotemporal functions of ciliary proteins. Leveraging existing -omics datasets can already offer some clues.
Unbiased systems-level studies to characterize ciliary networks
Recent high throughput approaches have enabled the identification of novel ciliary effectors using genome-wide screens performed in vitro (Table 1)[50–55••]. Small interfering (si)RNA-based functional genomics screens conducted in ciliated mammalian cell lines have focused primarily on identifying regulators of ciliogenesis, which in turn, led to the identification of novel ciliopathy genes[50,52,53]. These include INPP5E, mutations in which cause Joubert syndrome; and Agtpbp1, mutated in mice exhibiting ciliopathy-like phenotypes[50]. Further, a combinatorial approach of cell-based genome-wide screening and whole exome sequencing identified a genetic cause for approximately 5% of unexplained Joubert syndrome cases by uncovering mutations in KIAA0586, a gene known to be important for ciliogenesis and Hh signaling[52]. Given their unbiased approach, genome-wide screens have enabled the discovery of enriched groups of proteins with unexpected or poorly understood functions in ciliogenesis, including components of neuroactive G-protein-coupled receptors, the ubiquitin-proteasome system, and pre-mRNA splicing factors[53].
Table 1:
Overview of ciliary-focused functional genomic screens in mammalian cells
| Publication | Method | Cell type | Phenotypic readout | Hits | Validation approach | Core findings |
|---|---|---|---|---|---|---|
| Kim et al., 2010 | RNAi on 7,784 therapeuticallyrelevant genes | hTERT-RPE1 | Cilia number and length: EGFP-Smo reporter | 40 positive modulators and 13 negative modulators of ciliogenesis (12 ciliome proteins) | Confirmation screen at optimal cell density; verification of effect on cilia length; functional studies |
|
| Lai et al., 2011 | siRNA on 40 uncharacterized ciliary proteome genes and high-content analysis | S12; NIH/3T3; mIMCD3 | Cilia length, number, and transport of ciliary cargo: Gli3 luciferase and GFP-HTR6 reporters | 27 genes with impaired Hh signaling with or without cilia formation, elongation, or transport defects | Validated 12 strongest hits from primary screen; correlated mRNA levels with Hh signaling defects | 7 validated hits:
|
| Roosing et al., 2015 | Genome-wide siRNA (18,045 genes; (Dharmacon library; 4–5 siRNAs/gene) | hTERT-RPE1 | Cilia number and length: EGFP-Smo reporter; co-transfected nuclear cell cycle reporter (mCherry-Geminin) | 591 likely regulators of ciliogenesis | Combined cell-based screen with WES (145 individuals with unexplained JBTS) |
|
| Wheway et al., 2015 | Genome-wide siRNA (19,059 genes; ThermoFisher siGENOME library) | hTERT-RPE1; mIMCD3 | Loss of cilium: acetylated tubulin immunostaining | 68 validated genes involved in ciliogenesis in mIMCD3 cells; 37 validated genes in hTERT-RPE1 cells | qPCR, Western-blot, immunostaining, cross-comparison of hits with WES data (JBTS and JATD), in vivo validation in zebrafish |
|
| Pusapati et al., 2018 | Genome-wide CRISPR library (Brie library) | NIH/3T3-CG | Quantitative assessment of Hh signaling: GLI-GFP reporter | 641 genes: 40% of known Hh genes, 20% of all known cilia genes, 30% of known ciliopathy genes | Clonal knockout analysis in NIH/3T3 and NPC derived from mESC for 11 top regulators |
|
| Breslow et al., 2018 | Genome wide CRISPR (10 sgRNAs/gene) | NIH/3T3-CG | Assessment of Hh signaling: Gli:BlastR reporter conferring blasticidin resistance | 472 genes affecting Hh signaling (10% FDR) | Clonal knockout analysis for 6 new genes, immunofluorescence, mass spectrometry, luciferase assay |
|
High-throughput screening platforms interrogating cilia-mediated signaling, especially Hh, have also been adapted to utilize a CRISPR-based genome-wide knockout approach[54••,55••]. Not surprisingly, such screens[54••,55••] identified known modulators of Hh signaling, known ciliopathy genes, and validated ciliary components. In addition, both screens also uncovered: (1) novel positive and negative regulators of ciliogenesis, including the negative regulator, Atthog; (2) new ciliary components, including FAM92A as a transition-zone protein; and (3) a novel Meckel-Gruber Syndrome gene, TXNDC15, with mutations identified in a follow-up study in additional families with Meckel-Gruber Syndrome[56]. Surprisingly, within the 1,000 significant hits (20% false discovery rate) from the Breslow et al.[55••] screen using NIH 3T3 fibroblasts, there was an unexpected absence of kidney-related ciliopathy genes identified as significant hits, suggesting a potentially divergent mechanism specific to kidney-related ciliopathies in comparison to other ciliary phenotypes[57]. This observation highlights the need to conduct additional screens targeting both other pathways known to converge on cilia, such as Wnt, Hippo, and Notch, but also to consider executing these assays in different cell types. Indeed, it would be informative to re-run the published screens on a different cell type and ask what the divergence of hits might intimate about cell-specificity of function.
Proteomics studies to characterize networks within and beyond the cilium
Merging multiple independent ciliary and basal body proteomic studies identified the constituent proteins required to build and maintain a cilium[58]. However, the ciliome list lacks a spatially oriented map of the overall ciliary architecture with the added dimension of protein-protein interactions. Recently, systems-level analyses have provided a detailed ciliary interactome map depicting the molecular architecture of ciliary networks[39••,59,60]. These analyses used proximity labeling techniques, such as proximity-dependent biotinylation (BioID), tandem affinity purification (TAP) tagging, and a cilia-targeted proximity labeling enzyme (cilia-APEX), to label known centrosomal and ciliary proteins as “bait” for capturing protein interactions and coupled this affinity proteomics with mass spectrometry. In particular, >7,000 interactions were shown to comprise the centrosome-cilium interface[59] and ~5,000 interactions with clustering in 52 complexes were found in the ciliary landscape[39••]. A study by Boldt et al.[39••] highlighted the power of this analysis by: (1) finding >500 proteins not known to have a ciliary role interacting with a known ciliary protein; (2) achieving high resolution of sub-complexes that may have important implications for ciliary disease and structural biology; and 3) uncovering a potential disease association between 3M syndrome and ciliary dysfunction.
By necessity, existing protein-protein interaction data have focused on proteins with a known role in cilia as interactome baits[39••,59,60]. One approach to extend protein-protein interaction networks within and further beyond the cilium is to mine the Human Reference Protein Interactome Mapping Project (HuRI), established to systematically identify global binary protein-protein interactions using a yeast two-hybrid approach as the primary screening method [61]. An advantage to this approach is that it identifies binary protein-protein interactions in a non-organelle-specific manner. This is important because there is a growing appreciation that ciliary proteins also localize to extraciliary subcellular compartments, including the nucleus, the Golgi apparatus, and immune synapses of T cells[62–68].
To assess the usefulness of pan-cellular proteomics data in understanding further the complexity of ciliary networks, we used HuRI network data to query components of IFT complex B. Protein synthesis is not known to take place at the cilium, thus IFT is an evolutionarily conserved system that accomplishes bi-directional transport of ciliary cargo along the ciliary axoneme[69]. Further, IFT is critical for ciliary assembly and maintenance; with an anterograde complex (IFT-B) moving cargo towards the ciliary tip and a retrograde complex (IFT-A) recycling components to the ciliary base[70].
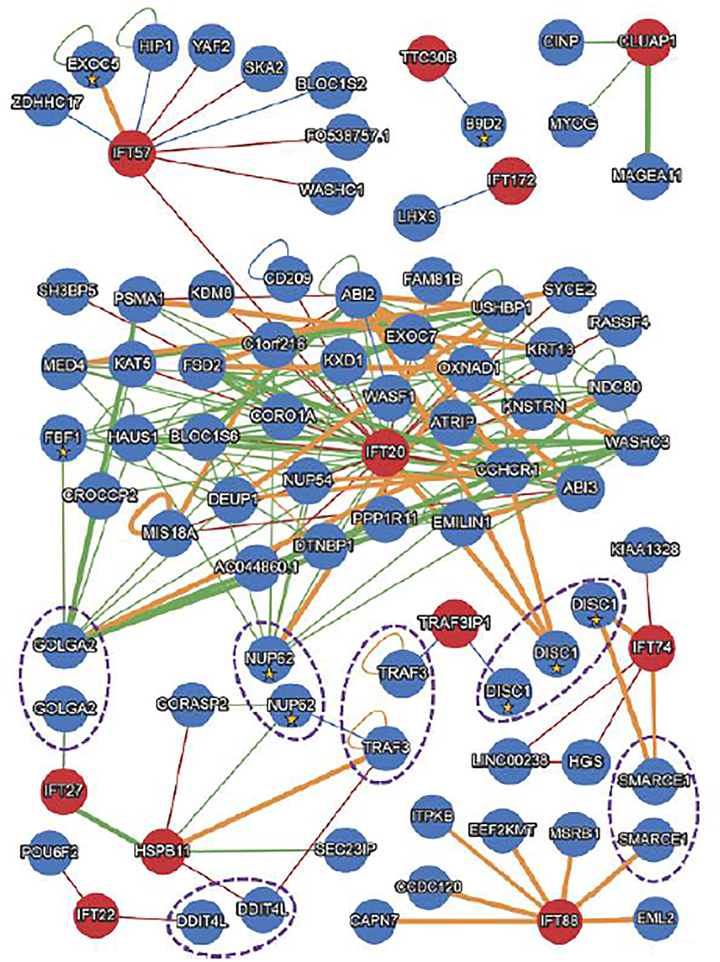
We analyzed all available IFT-B components (IFT20, IFT22, IFT25/HSPB11, IFT27, IFT38/CLUAP1, IFT54/TRAF3IP1, IFT57, IFT70/TTC30B, IFT74, IFT88, and IFT172) in HuRI (Figure 1), conceding that the extent of interactions will likely not be captured fully, due to the current unavailability for IFT46, IFT52, IFT80, and IFT81 network data. Nonetheless, we captured 67 total interactions of which there are six first-order shared interactors (DDIT4L, DISC1, GOLGA2, NUP62, SMARCE1 and TRAF3). Of the 67 interactors, 62 are not known to be ciliary; four are associated with the cilium, but also other subcellular sites (DISC1, EXOC5, FBF1, NUP62)[66,71,72]; and one (B9D2) is associated with autosomal recessive ciliopathies (Meckel-Gruber Syndrome and Joubert syndrome)[73,74]. The protein interaction networks across IFTB components are variable in the number of interactors and display minimal overlap of shared interactors, suggesting that: (1) the canonical viewpoint, based primarily on biochemical studies, that these components reside in a restricted complex might be oversimplified[75]; (2) the expansiveness of a protein network might reflect ciliary and non-ciliary roles for a given IFT protein, a posit already supported by a non-ciliary role for IFT88 in cell migration[76]; and (3) the composition of the IFT-B complex potentially influences protein interactions and biological function.
FIGURE 1: HuRI-generated protein-protein interaction networks for IFT-B components.
The Human Reference Protein Interactome (HuRI) Mapping Project (http://interactome.baderlab.org/) identifies binary protein-protein interactions using yeast two-hybrid as the primary screening method. For the interaction networks, the red circles denote the analyzed IFT-B members while the blue circles indicate interacting proteins. Interactors with known ciliary associations are denoted with a yellow star. Interactions are characterized with different colored lines indicating variable levels of validation: green lines (published interaction; at least two pieces of experimental evidence of which at least one stems from a binary interaction detection assay), orange lines (validated interaction; interactions that are not yet published but originate from datasets that have been validated by successfully testing a representative subset of these interactions in at least one orthogonal binary interaction detection assay), red lines (verified interaction; interactions that are neither published nor validated but were identified in pooled yeast two-hybrid (Y2H) screens, tested positive in Y2H pairwise test and sequence confirmed), or blue lines (interaction found in literature). The thickness of the line indicates the strength of the interaction between two proteins. Dashed purple ellipses indicate shared interactors between IFT-B members.
The protein interaction network for IFT20 supports these possibilities. It is heavily enriched with 40 interactions (versus a range between 1–9 interactions for all other proteins assessed; Figure 1) and includes a first-order interaction with IFT57, which is known to interact with IFT20 within the IFT-B complex and at immune synapses[77,78•]. In addition, we noted a first-order interaction between IFT20 and the cis-Golgi component GOLGA2/GM130. In support of biologically relevant non-ciliary interactions, a study by Stoetzel et al.[79•] highlighted formation of a complex between VPS15 and GOLGA2/GM130 that modulates IFT20-dependent trafficking from the cis-Golgi network to the primary cilium. It is not known whether IFT20 interacts directly with this complex in vivo but HuRI networks suggest that this might be so. Still, a caveat of HuRI data is that the subcellular location of interactions within cellular compartments is unknown, therefore it is not possible to differentiate between ciliary and non-ciliary interactions.
Using immunoprecipitation and affinity proteomics, recent studies have determined that the architecture of the IFT-B complex in mammals can be divided further into two evolutionarily conserved subcomplexes, IFT-B1 (core subcomplex) and IFT-B2 (peripheral subcomplex)[39••,80,81••]. Strong interactions between all IFT-B proteins within a subcomplex were not observed in these studies with a limited network of interactions present between subcomplexes[81••]. Consistent with these findings, we identified only two HuRI interactions between IFT proteins of the same subcomplex, IFT20-IFT57 (IFT-B2/peripheral subcomplex) and IFT27/HSPB11 (IFTB1/core subcomplex), both of which were robust using other methods[39••,81••]. Notably, IFT20 is considered a peripherally-associated IFT lying outside of the core subcomplex; we speculate that this potentially confers the ability to perform both nonciliary and ciliary functions[62,77,81••].
Unique spatiotemporal roles of ciliary proteins
Compared to our improved understanding of the protein composition of cilia, our understanding of how ciliary proteins function in diverse tissue types and timepoints remains rudimentary. Although the roles of primary cilia in development have been well studied, with emphasis on signaling pathways[1,15•], there have been fewer studies on the role of cilia in homeostasis[82] and regeneration[83]. Crucially, accumulating evidence highlights the transient presence of cilia that differs across tissue types and within constrained windows of time.
Cells within certain organs have been shown to be ciliated during development and then to become non-ciliated. For example, during aortic valve development in mice primary cilia have been shown: (1) to be spatially restricted to certain extracellular matrix zones within the aortic valve; and (2) to be present transiently on aortic valve mesenchymal cells starting at embryonic day (E)11.5 but lost postnatally[84•]. Another example of a transiently ciliated tissue is the kidney; immature rat podocytes are ciliated during glomerular development but cilia are not detectable in adult kidney podocytes[85]. This is potentially relevant to the variable severity and onset of some ciliopathy-causing loci such as the IFT-complex A gene, TTC21B, implicated in renal cystic kidney disease or progressive focal segmental glomerulosclerosis, despite the reported lack of cilia in adult podocytes[45,46].
The temporally-restricted appearance of cilia is not unique to early development. Some tissues have been shown to exhibit transient ciliation during differentiation. For instance, primary cilia are required to transduce Hh signaling during adipogenesis but only in differentiating preadipocytes; cilia are absent in both preadipocytes that are sub-confluent in cell culture and also in mature adipocytes[86]. In the olfactory epithelium, olfactory sensory neurons can regenerate after injury through a pool of basal stem cells[83,87]. Specifically, quiescent horizontal basal cells have a single, short primary cilium (about 4 μm) that is thought to detect lesions, triggering horizontal basal cells differentiation into multiciliated olfactory sensory neurons with elongated (50–60 μm) cilia with a wide range of olfactory receptors on their tips.
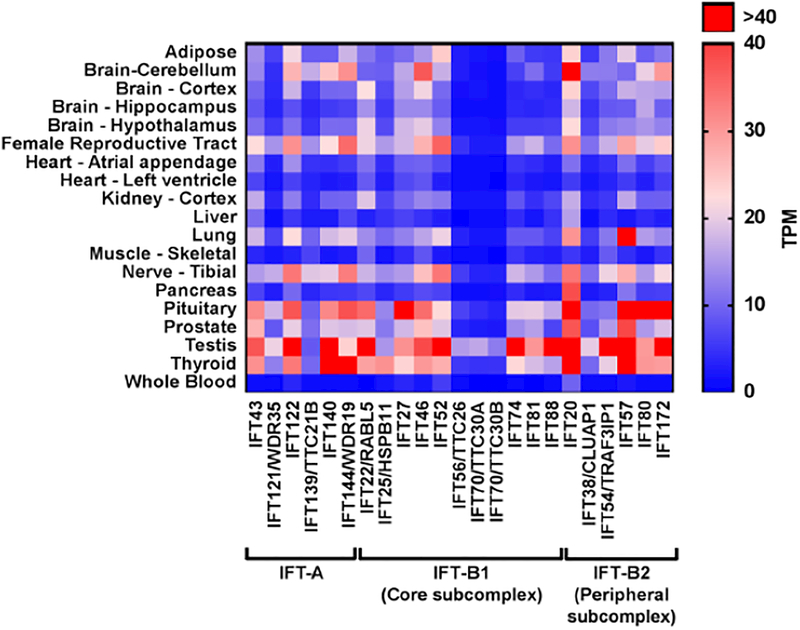
To explore the notion that tissue-specific differences might correlate with unique ciliary functions, we wondered whether there was differential expression of “core” ciliary proteins across tissues, with a continued emphasis on IFT. The IFT-A and -B members are crucial for ciliogenesis and maintenance, therefore their expression across tissues should correlate with ciliary distribution and abundance. Moreover, given that members of the same complex are dependent on each other to accomplish their function, one would expect that their expression (and variance) should be uniform within the same tissue type. As an initial test of these ideas, we collated gene expression data from all 23 known IFT genes across adult human tissues from the publicly available Genotype-Tissue Expression (GTEx) project database[88] based on transcript per million (TPM) values (Figure 2).
FIGURE 2: IFT-A and IFT-B gene expression in different adult tissues.
The heat map shows tissue-specific gene expression for IFT-A and IFT-B components (subcomplex IFT-B1 and IFT-B2) across differentiated human adult tissues. Raw transcript per million (TPM) values, representing gene expression values, were obtained from the GTEx database (https://www.gtexportal.org/home/). Red indicates higher expression (high TPM value) and blue indicates low expression (low TPM value), as reflected in the color gradient legend (right).
First, we were struck by the observation that the average gene expression across all tissues for IFT members within the same complex (A versus A; B versus B) is not uniform, with more than three-fold higher expression for IFT122 (25 TPM) versus IFT121 (7.1 TPM) in IFT-A and a more than twelve-fold difference between IFT20 (28.7 TPM) and IFT70/TTC30B (2.3 TPM) in IFT-B. Notably, two members of the IFTB complex, IFT20 and IFT57, show the highest average expression and were found to interact in multiple studies and within the HuRI network data[39••]. This observation for IFT20 potentially reflects both ciliary and non-ciliary IFT20, correlating with evidence for unique extraciliary localization of IFT20 in the Golgi apparatus and in the immune synapses of T cells[62,77].
Second, we observed marked differences in IFT gene expression across tissues. The lowest average IFT transcript abundance was reported in whole blood, with generally low expression levels (<4 TPM) across IFT components, with the exception of moderate expression in IFT20 (9.7 TPM). Hematopoietic cells are thought to lack cilia, despite studies which found primary cilia to be capable of responding to Hh ligands in cultured blood cells[89]. This is confounded by the non-ciliary role for IFT20 in immune synapse formation, suggesting that GTEx may be detecting nonciliary IFT20 in whole blood[67]. By contrast, the testes showed the highest average IFT gene expression (46.4 TPM) of any tissue, which was expected, given the essential function for IFTs in acrosome and flagella formation during spermatogenesis [90,91]. Consistent with this notion, tissues known to be multiciliated exhibited moderate to high expression, including the lungs (14.8 TPM) and the female reproductive tract (20.5 TPM). Notably, the thyroid and pituitary glands had the second highest average level of expression (both at 26.7 TPM). This is surprising because both glands are known to be ciliated but the role of cilia in these tissues is poorly understood[92,93]. Accordingly, the pituitary and thyroid glands also appeared in the top ten tissues expressing signature ciliary genes in an in silico analysis of microarray datasets across 104 normal human tissues[94].
Cilia were first observed in the human thyroid gland in 1988[92] but there are a paucity of subsequent studies, with the exception of a report indicating that cilia are present in most mammalian thyrocytes (but are notably absent in rat and mice thyrocytes)[95]. Regardless of the apparent lack of cilia in mouse thyrocytes, kinesin-2 mutant mice (Kif3aΔ/flox; Pax8Cre/+) have hypothyroidism and kidney cysts[96]. Hypothyroidism in ciliary mutants could intimate a role for the thyroid gland in the prevalent obesity phenotype observed in some ciliopathies. Also, the thyroid functions to maintain proper leptin signaling, along with the pituitary gland and hypothalamus, which has been implicated in obesity and is potentially important for ciliary biology[97].
We recognize that restricted mining of GTEx data to include a ciliary module at adult timepoints is coarse and we also do not know the potential correlation between protein abundance relative to its cognate mRNA. Certain tissues are only transiently ciliated, and moreover, proper ciliary function is essential for embryogenesis. Thus, there is a critical need to generate gene expression data, similar to what is available in the GTEx database, but during different developmental timepoints and from different cell populations within organs (e.g. renal tubule cells versus podocytes), potentially at the single cell level. A greater resolution of gene expression patterns is required to understand comprehensively why specific organ systems are differentially susceptible to deleterious mutations in ciliary genes, while measurement of ciliary protein abundance and subcellular distribution on a tissue by tissue basis will likewise be critical to improve resolution.
Concluding remarks and future directions
During a remarkable two decades of rapid progress, a vibrant research community has transformed our understanding of the primary cilium from a mechanical “oar” or even a vestigial organelle to a signaling hub critical for a vast array of developmental and homeostatic processes. Concomitant to these advances was the appreciation that ciliary disorders represent a significant burden, while at the same time allowed us to begin to gleam insights into one of the most challenging facets of human genetics: variable expressivity. At present, we have a reasonable structural and biochemical representation of the “generic” cilium. At the same time, we are beginning to understand that this emergent picture is a coarse representation of biological reality and that the complexity and diversity of this organelle is much greater than we might have anticipated. In that regard, the continuous, now systematic amassment of ciliary proteins might need to start considering the cilium as a dynamic organelle, with proteins interacting within complexes and between functional networks. With the advent of genome-wide screens and increasingly sophisticated affinity proteomics strategies, it is now possible to deploy unbiased screens with greater sensitivity and specificity to capture interactions both within the cilium and extending to additional cellular compartments. At the same time, it is important to temper “ciliophilia”, where the observation of a protein that either has interesting functions or is mutated in human disease de facto expands both the ciliopathies and the functions of the organelle. In that context, a major challenge persists in that we have poor tools to understand functions that are ciliary-dependent versus ciliary independent versus ciliary-related but not occurring at the cilium[65,98•]. We are beginning to gain clues about ciliary trafficking and signal sequences that govern some of them[99]. It might be useful to generate appropriate mutants, in vitro and in vivo and study the biochemical properties and phenotypic consequences of molecules that retain their non-ciliary functions.
Moving forward, the emergent appreciation of the spatiotemporal complexity and likely fine regulation of ciliary function will undoubtedly assist our understanding of phenotypic variability in ciliopathies. In that context, continued multi-dimensional mapping of ciliary composition will be critical. Expanding from the highly artificial handful of cell types in which ciliary biology and composition has been studied, systematic imaging, transcriptomic, and proteomic studies across tissue types, developmental time points and key homeostatic junctures are likely to be highly informative. We look forward to a future image of the organelle composed of ciliary networks (both at the level of mRNA regulation and protein composition/trafficking/signaling) layered with human variation and longitudinal clinical data. Such constructs will be able to better capture both individual protein variability (isoforms with subcellular or tissue-specific expression) and spatiotemporal contexts (developmental and adult stages). Finally, it will be important to ask if there is inter-human variability in ciliary composition and function. Given the complexity of the organelle, it is not hard to imagine that humans will have both stochastic and genetically defined differences in the function of cilia in some cellular sites.
The rapid advances of ciliary biology and the concomitant discovery of the ciliopathies have had a profound impact on our understanding of human biology and genetics. Some two decades later, the community is poised to harness newer conceptual and technological tools and use this organelle and its allied human disorders as a paradigm of systems biology, wherein the composition and distribution of the systems components are defined mapped and racked in relevance with human pathology. Such studies will not only expedite discovery and therapeutics but will also generate functional models useful for a host of other biological systems and disease groups.
ACKNOWLEDGEMENTS
We thank the members of the Duke Center for Human Disease Modeling for thoughtful discussions and we apologize to our colleagues whose work could not be cited in this review due to space constraints. This work was supported by U.S. National Institutes of Health grants HD042601, DK072301 and GM121317. N.K. is a Distinguished Jean and George Brumley Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST. N.K. is a paid consultant for and holds significant stock of Rescindo Therapeutics, Inc.
REFERENCES
- 1.Goetz SC, Anderson KV: The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010, 11:331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, Katsanis N: The vertebrate primary cilium in development, homeostasis, and disease. Cell 2009, 137:32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••3.Reiter JF, Leroux MR: Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol 2017, 18:533–547.This comprehensive review gives a broad overview of the diversity of ciliopathies and reflects on non-ciliary roles of ciliary proteins.
- 4.Satir P: Landmarks in cilia research from Leeuwenhoek to us. Cell Motil Cytoskeleton 1995, 32:90–94. [DOI] [PubMed] [Google Scholar]
- 5.Worthington WC Jr., Cathcart RS 3rd: Ependymal cilia: distribution and activity in the adult human brain. Science 1963, 139:221–222. [DOI] [PubMed] [Google Scholar]
- 6.Tilley AE, Walters MS, Shaykhiev R, Crystal RG: Cilia dysfunction in lung disease. Annu Rev Physiol 2015, 77:379–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitfield TT: Development of the inner ear. Curr Opin Genet Dev 2015, 32:112–118. [DOI] [PubMed] [Google Scholar]
- 8.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N: Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 1998, 95:829–837. [DOI] [PubMed] [Google Scholar]
- 9.Bloodgood RA: From central to rudimentary to primary: the history of an underappreciated organelle whose time has come. The primary cilium. Methods Cell Biol 2009, 94:3–52. [DOI] [PubMed] [Google Scholar]
- 10.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG: Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 2000, 151:709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrick JJ, Onuchic LF, Reeders ST, Korenberg J, Chen XN, Moyer JH, Wilkinson JE, Woychik RP: Characterization of the human homologue of the mouse Tg737 candidate polycystic kidney disease gene. Hum Mol Genet 1995, 4:559–567. [DOI] [PubMed] [Google Scholar]
- 12.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV: Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003, 426:83–87. [DOI] [PubMed] [Google Scholar]
- 13.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. : Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 2003, 33:129–137. [DOI] [PubMed] [Google Scholar]
- 14.Singla V, Reiter JF: The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 2006, 313:629–633. [DOI] [PubMed] [Google Scholar]
- •15.Wheway G, Nazlamova L, Hancock JT: Signaling through the Primary Cilium. Front Cell Dev Biol 2018, 6:8.This review summarizes the diverse signaling pathways that function through the primary cilium and associates ciliopathies with underlying signaling defects.
- 16.Afzelius BA: A human syndrome caused by immotile cilia. Science 1976, 193:317–319. [DOI] [PubMed] [Google Scholar]
- 17.Olbrich H, Fliegauf M, Hoefele J, Kispert A, Otto E, Volz A, Wolf MT, Sasmaz G, Trauer U, Reinhardt R, et al. : Mutations in a novel gene, NPHP3, cause adolescent nephronophthisis, tapeto-retinal degeneration and hepatic fibrosis. Nat Genet 2003, 34:455–459. [DOI] [PubMed] [Google Scholar]
- 18.Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D, et al. : Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left-right axis determination. Nat Genet 2003, 34:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, et al. : Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature 2003, 425:628–633. [DOI] [PubMed] [Google Scholar]
- 20.Badano JL, Mitsuma N, Beales PL, Katsanis N: The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 2006, 7:125–148. [DOI] [PubMed] [Google Scholar]
- 21.Tobin JL, Beales PL: The nonmotile ciliopathies. Genet Med 2009, 11:386–402. [DOI] [PubMed] [Google Scholar]
- 22.Davis EE, Katsanis N: The ciliopathies: a transitional model into systems biology of human genetic disease. Curr Opin Genet Dev 2012, 22:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satir P, Christensen ST: Overview of structure and function of mammalian cilia. Annu Rev Physiol 2007, 69:377–400. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro AJ, Leigh MW: Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure. Ultrastruct Pathol 2017, 41:373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feistel K, Blum M: Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn 2006, 235:3348–3358. [DOI] [PubMed] [Google Scholar]
- 26.Silverman MA, Leroux MR: Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol 2009, 19:306–316. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno N, Taschner M, Engel BD, Lorentzen E: Structural studies of ciliary components. J Mol Biol 2012, 422:163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC: A proteomic analysis of human cilia: identification of novel components. Mol Cell Proteomics 2002, 1:451–465. [DOI] [PubMed] [Google Scholar]
- 29.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M: Proteomic characterization of the human centrosome by protein correlation profiling. Nature 2003, 426:570–574. [DOI] [PubMed] [Google Scholar]
- 30.Avidor-Reiss T, Maer AM, Koundakjian E, Polyanovsky A, Keil T, Subramaniam S, Zuker CS: Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 2004, 117:527–539. [DOI] [PubMed] [Google Scholar]
- 31.Li JB, Gerdes JM, Haycraft CJ, Fan Y, Teslovich TM, May-Simera H, Li H, Blacque OE, Li L, Leitch CC, et al. : Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 2004, 117:541–552. [DOI] [PubMed] [Google Scholar]
- 32.Pazour GJ, Agrin N, Leszyk J, Witman GB: Proteomic analysis of a eukaryotic cilium. J Cell Biol 2005, 170:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stolc V, Samanta MP, Tongprasit W, Marshall WF: Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc Natl Acad Sci U S A 2005, 102:3703–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blacque OE, Perens EA, Boroevich KA, Inglis PN, Li C, Warner A, Khattra J, Holt RA, Ou G, Mah AK, et al. : Functional genomics of the cilium, a sensory organelle. Curr Biol 2005, 15:935–941. [DOI] [PubMed] [Google Scholar]
- 35.Efimenko E, Bubb K, Mak HY, Holzman T, Leroux MR, Ruvkun G, Thomas JH, Swoboda P: Analysis of xbx genes in C. elegans. Development 2005, 132:1923–1934. [DOI] [PubMed] [Google Scholar]
- 36.Inglis PN, Boroevich KA, Leroux MR: Piecing together a ciliome. Trends Genet 2006, 22:491–500. [DOI] [PubMed] [Google Scholar]
- 37.Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, et al. : Flagellar motility is required for the viability of the bloodstream trypanosome. Nature 2006, 440:224–227. [DOI] [PubMed] [Google Scholar]
- 38.van Dam TJ, Wheway G, Slaats GG, Group SS, Huynen MA, Giles RH: The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2013, 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••39.Boldt K, van Reeuwijk J, Lu Q, Koutroumpas K, Nguyen TM, Texier Y, van Beersum SE, Horn N, Willer JR, Mans DA, et al. : An organelle-specific protein landscape identifies novel diseases and molecular mechanisms. Nat Commun 2016, 7:11491.This systems-level study coupled mass spectrometry with tandem affinity proteomics to capture a ciliary protein landscape with detailed networks of interactions, including >500 interactions between ciliary and suspected non-ciliary proteins. By using 217 proteins associated with ciliary function or disease, the authors resolved previously unidentified networks of interactions, subcomplexes of ciliary modules, and a novel disease association with cilia and 3M syndrome.
- 40.Ishikawa H, Thompson J, Yates JR 3rd, Marshall WF: Proteomic analysis of mammalian primary cilia. Curr Biol 2012, 22:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. : A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007, 129:1201–1213. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. : A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 2011, 43:776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia G 3rd, Raleigh DR, Reiter JF: How the Ciliary Membrane Is Organized Inside-Out to Communicate Outside-In. Curr Biol 2018, 28:R421–R434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, et al. : Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 2010, 329:1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huynh Cong E, Bizet AA, Boyer O, Woerner S, Gribouval O, Filhol E, Arrondel C, Thomas S, Silbermann F, Canaud G, et al. : A homozygous missense mutation in the ciliary gene TTC21B causes familial FSGS. J Am Soc Nephrol 2014, 25:2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bullich G, Vargas I, Trujillano D, Mendizabal S, Pinero-Fernandez JA, Fraga G, Garcia-Solano J, Ballarin J, Estivill X, Torra R, et al. : Contribution of the TTC21B gene to glomerular and cystic kidney diseases. Nephrol Dial Transplant 2017, 32:151–156. [DOI] [PubMed] [Google Scholar]
- 47.Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV, et al. : TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 2011, 43:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer E, Stoetzel C, Scheidecker S, Geoffroy V, Prasad MK, Redin C, Missotte I, Lacombe D, Mandel JL, Muller J, et al. : Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet-Biedl syndrome. J Hum Genet 2016, 61:447–450. [DOI] [PubMed] [Google Scholar]
- 49.Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C, et al. : Mutations in IFT172 cause isolated retinal degeneration and Bardet-Biedl syndrome. Hum Mol Genet 2015, 24:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG: Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010, 464:1048–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai CK, Gupta N, Wen X, Rangell L, Chih B, Peterson AS, Bazan JF, Li L, Scales SJ: Functional characterization of putative cilia genes by high-content analysis. Mol Biol Cell 2011, 22:1104–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roosing S, Hofree M, Kim S, Scott E, Copeland B, Romani M, Silhavy JL, Rosti RO, Schroth J, Mazza T, et al. : Functional genome-wide siRNA screen identifies KIAA0586 as mutated in Joubert syndrome. Elife 2015, 4:e06602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wheway G, Schmidts M, Mans DA, Szymanska K, Nguyen TT, Racher H, Phelps IG, Toedt G, Kennedy J, Wunderlich KA, et al. : An siRNA-based functional genomics screen for the identification of regulators of ciliogenesis and ciliopathy genes. Nat Cell Biol 2015, 17:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••54.Pusapati GV, Kong JH, Patel BB, Krishnan A, Sagner A, Kinnebrew M, Briscoe J, Aravind L, Rohatgi R: CRISPR Screens Uncover Genes that Regulate Target Cell Sensitivity to the Morphogen Sonic Hedgehog. Dev Cell 2018, 44:113–129 e118.This genome-wide CRISPR-based screen in mouse fibroblasts (NIH/3T3-CG cells) identified candidate modulators of hedgehog (Hh) signaling. In total, the authors found 641 genes altering Hh signaling capacity, including 20% of all known ciliary genes and 30% of known ciliopathy genes. Atthog was characterized as a novel negative regulator of Hh signaling, affecting the stability of the important Hh component, smoothened.
- ••55.Breslow DK, Hoogendoorn S, Kopp AR, Morgens DW, Vu BK, Kennedy MC, Han K, Li A, Hess GT, Bassik MC, et al. : A CRISPR-based screen for Hedgehog signaling provides insights into ciliary function and ciliopathies. Nat Genet 2018, 50:460–471.The authors identified new ciliary and centriolar components as well as novel ciliopathy genes, including TXNDC15 as a novel Meckel syndrome gene, by deploying a genome-wide CRISPR screen in Hh-responsive mouse fibroblasts (NIH/3T3-CG cells) engineered to confer blasticidin resistance for antibiotic selection. There was a notable absence of kidney-related ciliopathy genes from this analysis, suggesting divergent signaling requirements for renal ciliopathies.
- 56.Shaheen R, Szymanska K, Basu B, Patel N, Ewida N, Faqeih E, Al Hashem A, Derar N, Alsharif H, Aldahmesh MA, et al. : Characterizing the morbid genome of ciliopathies. Genome Biol 2016, 17:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Otto EA: Is ciliary Hedgehog signalling dispensable in the kidneys? Nat Rev Nephrol 2018, 14:415–416. [DOI] [PubMed] [Google Scholar]
- 58.Gherman A, Davis EE, Katsanis N: The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet 2006, 38:961–962. [DOI] [PubMed] [Google Scholar]
- 59.Gupta GD, Coyaud E, Goncalves J, Mojarad BA, Liu Y, Wu Q, Gheiratmand L, Comartin D, Tkach JM, Cheung SW, et al. : A Dynamic Protein Interaction Landscape of the Human Centrosome-Cilium Interface. Cell 2015, 163:1484–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV: Proteomics of Primary Cilia by Proximity Labeling. Dev Cell 2015, 35:497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rolland T, Tasan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, et al. : A proteome-scale map of the human interactome network. Cell 2014, 159:1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Follit JA, Tuft RA, Fogarty KE, Pazour GJ: The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 2006, 17:3781–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K, et al. : Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 2014, 5:5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giorgio G, Alfieri M, Prattichizzo C, Zullo A, Cairo S, Franco B: Functional characterization of the OFD1 protein reveals a nuclear localization and physical interaction with subunits of a chromatin remodeling complex. Mol Biol Cell 2007, 18:4397–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gascue C, Tan PL, Cardenas-Rodriguez M, Libisch G, Fernandez-Calero T, Liu YP, Astrada S, Robello C, Naya H, Katsanis N, et al. : Direct role of Bardet-Biedl syndrome proteins in transcriptional regulation. J Cell Sci 2012, 125:362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McClure-Begley TD, Klymkowsky MW: Nuclear roles for cilia-associated proteins. Cilia 2017, 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finetti F, Paccani SR, Rosenbaum J, Baldari CT: Intraflagellar transport: a new player at the immune synapse. Trends Immunol 2011, 32:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finetti F, Onnis A, Baldari CT: Regulation of vesicular traffic at the T cell immune synapse: lessons from the primary cilium. Traffic 2015, 16:241–249. [DOI] [PubMed] [Google Scholar]
- 69.Rosenbaum JL, Witman GB: Intraflagellar transport. Nat Rev Mol Cell Biol 2002, 3:813–825. [DOI] [PubMed] [Google Scholar]
- 70.Ishikawa H, Marshall WF: Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol 2011, 12:222–234. [DOI] [PubMed] [Google Scholar]
- 71.Morris JA, Kandpal G, Ma L, Austin CP: DISC1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet 2003, 12:1591–1608. [DOI] [PubMed] [Google Scholar]
- 72.Sugimoto M, Inoko A, Shiromizu T, Nakayama M, Zou P, Yonemura S, Hayashi Y, Izawa I, Sasoh M, Uji Y, et al. : The keratin-binding protein Albatross regulates polarization of epithelial cells. J Cell Biol 2008, 183:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dowdle WE, Robinson JF, Kneist A, Sirerol-Piquer MS, Frints SG, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, et al. : Disruption of a ciliary B9 protein complex causes Meckel syndrome. Am J Hum Genet 2011, 89:94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bachmann-Gagescu R, Dempsey JC, Phelps IG, O’Roak BJ, Knutzen DM, Rue TC, Ishak GE, Isabella CR, Gorden N, Adkins J, et al. : Joubert syndrome: a model for untangling recessive disorders with extreme genetic heterogeneity. J Med Genet 2015, 52:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taschner M, Bhogaraju S, Vetter M, Morawetz M, Lorentzen E: Biochemical mapping of interactions within the intraflagellar transport (IFT) B core complex: IFT52 binds directly to four other IFT-B subunits. J Biol Chem 2011, 286:26344–26352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boehlke C, Janusch H, Hamann C, Powelske C, Mergen M, Herbst H, Kotsis F, Nitschke R, Kuehn EW: A Cilia Independent Role of Ift88/Polaris during Cell Migration. PLoS One 2015, 10:e0140378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vivar OI, Masi G, Carpier JM, Magalhaes JG, Galgano D, Pazour GJ, Amigorena S, Hivroz C, Baldari CT: IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proc Natl Acad Sci U S A 2016, 113:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •78.Galgano D, Onnis A, Pappalardo E, Galvagni F, Acuto O, Baldari CT: The T cell IFT20 interactome reveals new players in immune synapse assembly. J Cell Sci 2017, 130:1110–1121.This study used quantitative mass-spectrometry to identify new interactors of IFT20 in Jurkat T cells. IFT54, GMAP-210, ARPC3, COP9 and ERGIC-53 were shown to interact with IFT20 and to be required for proper T-cell antigen receptors trafficking to the immune synapse. IFT54 is the fourth member of the IFT-B complex to be shown to play a non-ciliary role at immune synapse.
- •79.Stoetzel C, Bar S, De Craene JO, Scheidecker S, Etard C, Chicher J, Reck JR, Perrault I, Geoffroy V, Chennen K, et al. : A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat Commun 2016, 7:13586.This study described a ciliopathy-associated mutation in VPS15 which was shown to alter cilia length (shorter cilia) in human fibroblasts. Mutant fibroblasts also exhibited aberrant IFT20 localization, with IFT20 restricted to the Golgi instead of within vesicles trafficking to the cilium. In vivo modeling in zebrafish revealed ciliopathy-like phenotypes in zebrafish depleted of vps15, supporting the important role for this gene in ciliary function and disease.
- 80.Bhogaraju S, Engel BD, Lorentzen E: Intraflagellar transport complex structure and cargo interactions. Cilia 2013, 2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••81.Katoh Y, Terada M, Nishijima Y, Takei R, Nozaki S, Hamada H, Nakayama K: Overall Architecture of the Intraflagellar Transport (IFT)-B Complex Containing Cluap1/IFT38 as an Essential Component of the IFT-B Peripheral Subcomplex. J Biol Chem 2016, 291:10962–10975.The authors developed an immunoprecipitation strategy for visualizing binary protein interactions with a fluorescence microscope instead of through immunoblotting, to resolve the overall architecture of the IFT-B complex. Using this method, they found two subcomplexes: a core subcomplex (10 subunits) and a peripheral subcomplex (6 subunits). Further, they identified two new subunits of the IFT-B complex, TTC26/IFT56 (core subcomplex) and Cluap1/IFT38 (peripheral subcomplex).
- 82.Croyle MJ, Lehman JM, O’Connor AK, Wong SY, Malarkey EB, Iribarne D, Dowdle WE, Schoeb TR, Verney ZM, Athar M, et al. : Role of epidermal primary cilia in the homeostasis of skin and hair follicles. Development 2011, 138:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Joiner AM, Green WW, McIntyre JC, Allen BL, Schwob JE, Martens JR: Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium. J Neurosci 2015, 35:13761–13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •84.Toomer KA, Fulmer D, Guo L, Drohan A, Peterson N, Swanson P, Brooks B, Mukherjee R, Body S, Lipschutz JH, et al. : A role for primary cilia in aortic valve development and disease. Dev Dyn 2017, 246:625–634.This is the first demonstration that aortic valve mesenchymal cells have spatially-restricted (within extracellular matrix zones), transient primary cilia during mouse embryogenesis that are lost postnatally. Given these findings, this study implicated primary cilia in bicuspid aortic valve disease.
- 85.Ichimura K, Kurihara H, Sakai T: Primary cilia disappear in rat podocytes during glomerular development. Cell Tissue Res 2010, 341:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H: Transient ciliogenesis involving Bardet-Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci U S A 2009, 106:1820–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DeMaria S, Ngai J: The cell biology of smell. J Cell Biol 2010, 191:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Consortium GT: The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013, 45:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Singh M, Chaudhry P, Merchant AA: Primary cilia are present on human blood and bone marrow cells and mediate Hedgehog signaling. Exp Hematol 2016, 44:1181–1187 e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kierszenbaum AL, Rivkin E, Tres LL, Yoder BK, Haycraft CJ, Bornens M, Rios RM: GMAP210 and IFT88 are present in the spermatid golgi apparatus and participate in the development of the acrosome-acroplaxome complex, head-tail coupling apparatus and tail. Dev Dyn 2011, 240:723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Liu H, Li W, Zhang Z, Shang X, Zhang D, Li Y, Zhang S, Liu J, Hess RA, et al. : Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev Biol 2017, 432:125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martin A, Hedinger C, Haberlin-Jakob M, Walt H: Structure and motility of primary cilia in the follicular epithelium of the human thyroid. Virchows Arch B Cell Pathol Incl Mol Pathol 1988, 55:159–166. [DOI] [PubMed] [Google Scholar]
- 93.Iwanaga T, Hozumi Y, Takahashi-Iwanaga H: Immunohistochemical demonstration of dopamine receptor D2R in the primary cilia of the mouse pituitary gland. Biomed Res 2011, 32:225–235. [DOI] [PubMed] [Google Scholar]
- 94.Ivliev AE, t Hoen PA, van Roon-Mom WM, Peters DJ, Sergeeva MG: Exploring the transcriptome of ciliated cells using in silico dissection of human tissues. PLoS One 2012, 7:e35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Utrilla JC, Gordillo-Martinez F, Gomez-Pascual A, Fernandez-Santos JM, Garnacho C, Vazquez-Roman V, Morillo-Bernal J, Garcia-Marin R, Jimenez-Garcia A, Martin-Lacave I: Comparative study of the primary cilia in thyrocytes of adult mammals. J Anat 2015, 227:550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Amico E, Gayral S, Massart C, Van Sande J, Reiter JF, Dumont JE, Robaye B, Schurmans S: Thyroid-specific inactivation of KIF3A alters the TSH signaling pathway and leads to hypothyroidism. J Mol Endocrinol 2013, 50:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Volta F, Gerdes JM: The role of primary cilia in obesity and diabetes. Ann N Y Acad Sci 2017, 1391:71–84. [DOI] [PubMed] [Google Scholar]
- •98.Abe S, Nagai T, Masukawa M, Okumoto K, Homma Y, Fujiki Y, Mizuno K: Localization of Protein Kinase NDR2 to Peroxisomes and Its Role in Ciliogenesis. J Biol Chem 2017, 292:4089–4098.This study demonstrated that wild-type NDR2 localizes to peroxisomes facilitated by a Leucine residue in the C-terminus of the protein, and that ciliogenesis defects observed in NDR2-depleted cells are caused by the loss of NDR2 peroxisome localization. This is an exemplar of a non-ciliary protein affecting ciliogenesis.
- 99.Schou KB, Pedersen LB, Christensen ST: Ins and outs of GPCR signaling in primary cilia. EMBO Rep 2015, 16:1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]