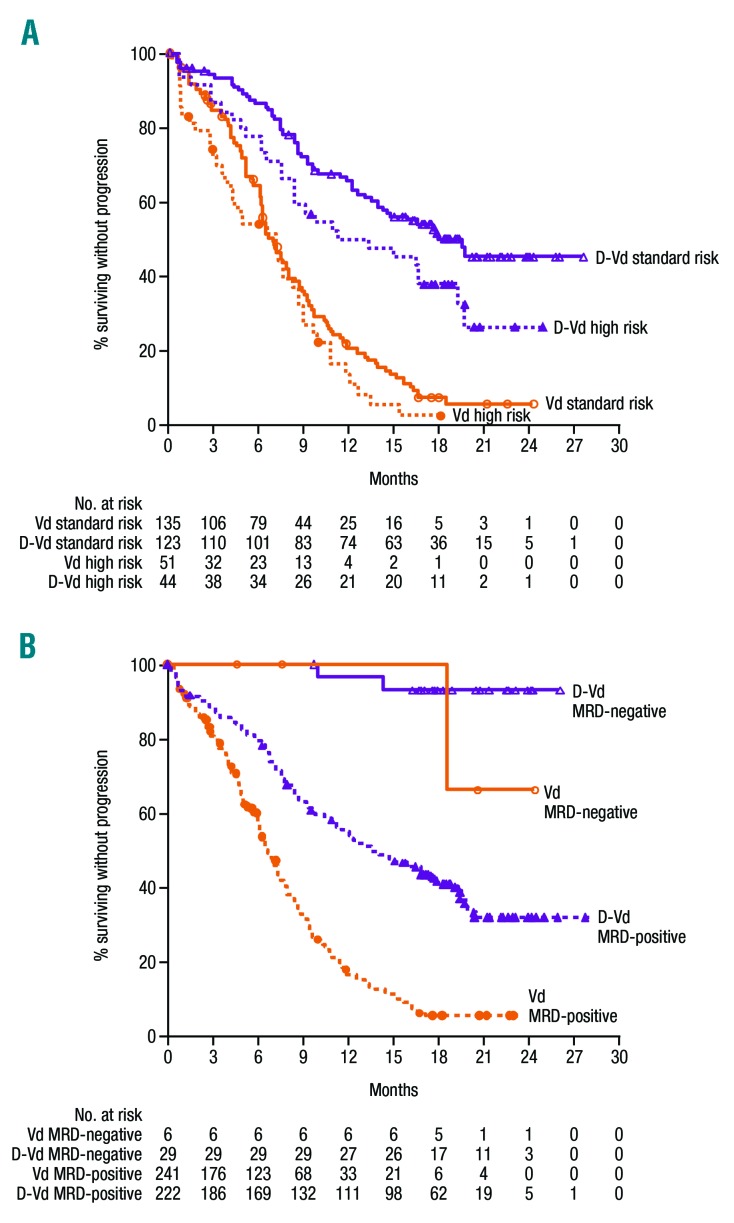
Figure 3.
PFS survival based on (A) cytogenetic risk and (B) MRD status. (A) Kaplan-Meier estimates of PFS among patients evaluated for cytogenetic risk. High-risk patients had any of t(4;14), t(14;16), or del17p cytogenetic abnormalities as determined by central next-generation sequencing. Standard-risk patients had an absence of high-risk abnormalities. (B) Kaplan-Meier estimates of PFS among patients in the ITT population population. MRD-negative status was evaluated at a sensitivity threshold of 10−5 using bone marrow aspirate samples that were prepared using Ficoll and analyzed by the clonoSEQ® assay. MRD: minimal residual disease; D-Vd: daratumumab plus bortezomib and dexamethasone; Vd: bortezomib and dexamethasone.