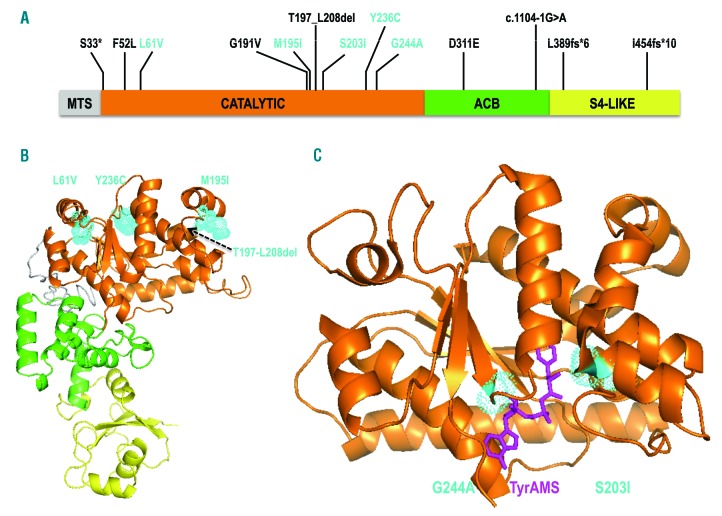
Figure 1.
Representation of mutated YARS2 proteins. (A) Schematic view of YARS2 domains: MTS: mitochondrial targeting sequence; ACB: anticodon binding domain; S4-Like: S4 ribosomal protein-like domain. Amongst all the variants identified, only those tested in this study are shown in cyan. Note that the recombinant YARS2 used in the amino-acylation assays is deprived of the MTS. (B) Model of YARS2 p.(Thr197-Leu208del), built with I-TASSER.28 The structural domains from (A) are shown with the same color code. The locations of the variants, which have the weakest effects on amino-acylation [p.(Leu61Val), p.(Met195Ile), p.(Tyr236Cys)] are shown in cyan. (C) Crystal structure of YARS2 catalytic domain19 with the tyrosyl-adenylate analog (TyrAMS, magenta) bound to the active site. The locations of variants p.(Ser203Ile) and p.(Gly244Ala), characterized by the strongest effects on amino-acylation, are indicated in cyan.