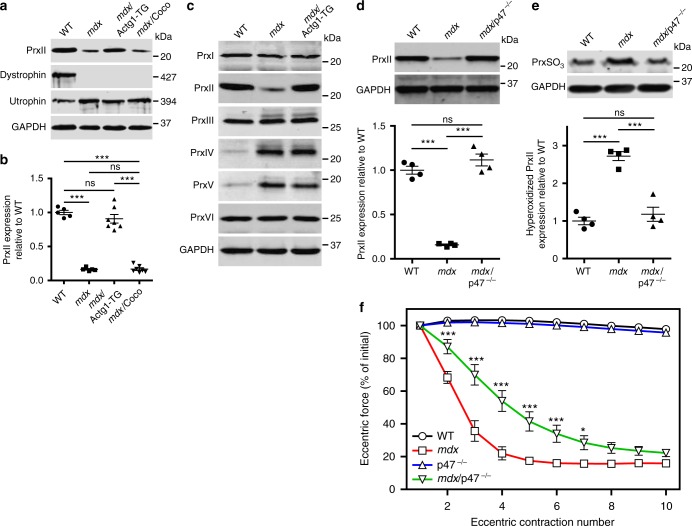
Fig. 2.
Peroxiredoxin-2 is significantly decreased in mdx skeletal muscle and restored by γcyto-actin overexpression and genetic ablation of NOX2 activity. a Immunoblot analysis of PrxII, dystrophin, utrophin, and GAPDH in WT, mdx, mdx/Actg1-TG, and mdx/Coco gastrocnemius muscles. b Immunoblot quantitation demonstrated that PrxII levels in mdx skeletal muscle were 16.5 ± 0.03% of WT and restored in mdx/Actg1-TG muscle to levels not different from WT, but not in mdx/Coco muscle; n = 5 for WT and mdx; n = 7 for mdxActg1-TG and mdx/Coco. ***P < 0.001, ns no significance; one-way ANOVA. c Immunoblot analysis of peroxiredoxins 1–6 in gastrocnemius muscles from WT, mdx, and mdx/Actg1-TG mice. PrxII was the only peroxiredoxin isoform that was both altered in mdx compared to WT, and also restored to its WT level by muscle-specific γcyto-actin overexpression. d Immunoblot analysis of PrxII in WT, mdx, and mdx/p47–/– gastrocnemius muscles demonstrated a restoration of PrxII to WT levels in mdx/p47–/– muscle; n = 4 for each genotype. ***P < 0.001, ns no significance; one-way ANOVA. e Immunoblot analysis demonstrated significantly elevated hyperoxidized peroxiredoxin (PrxSO3) in mdx compared to WT, and restored to WT levels in mdx/p47–/– gastrocnemius muscles; n = 4 for each genotype. ***P < 0.001, ns no significance; one-way ANOVA. f EDL muscles isolated from WT, mdx, p47–/–, and mdx/p47–/– mice were subjected to 10 eccentric contractions and the forces measured expressed as a percentage of the force generated during the first eccentric contraction; n = 4 for WT and mdx; n = 3 for p47–/–; n = 7 for mdx/p47–/–. *P < 0.05, ***P < 0.001 compared to mdx; two-way ANOVA. Throughout, error bars represent means ± SEM