Table 1.
Synthesis of cyclovertrylenes a.
| Entry | Alcohol | Products | Yield% b | ||||
|---|---|---|---|---|---|---|---|
| MW c | IR d | ||||||
| 1 | 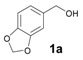 |
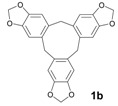 |
85 | 80 | |||
 |
1 | 3 | |||||
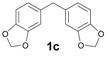
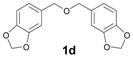 |
2 | 5 | |||||
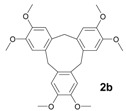
 |
4 | 5 | |||||
 |
8 | 7 | |||||
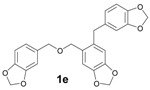
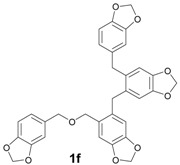
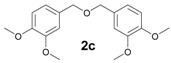
| 2 | 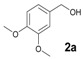 |
 |
90 | 88 | |||
 |
10 | 12 | |||||
| 3 | 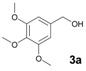 |
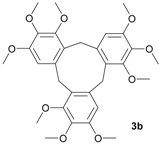 |
80 | 75 | |||
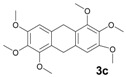 |
5 | 5 | |||||
 |
15 | 20 | |||||
a Reaction conditions: benzyl alcohols 1a–3a (2 mmol) and TAFF (20 mg); b Yield of isolated product after chromatographic purification; c Microwave at 85 °C (100W), 1.50–5 min; d IR at 95 °C (375W), 3–7 min.
