Table 2.
Synthesis of oligotoluenes using two different heating models: MW and IR a.
| Entry | Alcohol | Products | Yield% b | ||
|---|---|---|---|---|---|
| MW c | IR d | ||||
| 1 | 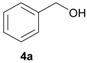 |
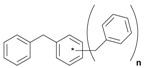 |
4b n = 0 | 0 | 0 |
| 4c n = 1 | 60 | 58 | |||
| 4d n = 2 | 40 | 22 | |||
| 4e n = 3 | 0 | 20 | |||
| 2 | 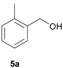 |
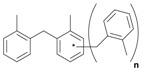 |
5b n = 0 | 0 | 0 |
| 5c n = 1 | 55 | 0 | |||
| 5d n = 2 | 28 | 0 | |||
| 5e n = 3 | 12 | 0 | |||
| 5f n = 4 | 5 | 0 | |||
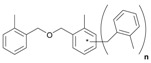 |
5g n = 0 | 0 | 65 | ||
| 5h n = 1 | 0 | 25 | |||
| 5i n = 2 | 0 | 10 | |||
| 3 | 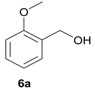 |
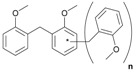 |
6b n = 0 | 0 | 0 |
| 6c n = 1 | 35 | 8 | |||
| 6d n = 2 | 65 | 92 | |||
a Reaction conditions: benzyl alcohols 4a–6a (2 mmol) and TAFF (20 mg); b Yields and composition of reaction mixture was determinate by GC-EIMS and HRMS; c Microwave at 85 °C (100 W), 4–10 min; d IR at 95 °C (375 W), 2.5–10 min.
