Table 1.
Optimization of reaction conditions a. 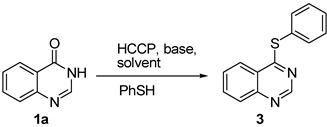
| Entry | Base | Solvent | Temperature (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | TEA | MeCN | 45 | 48 |
| 2 | K2CO3 | MeCN | 45 | 32 |
| 3 | Cs2CO3 | MeCN | 45 | 69 |
| 4 | DIPEA | MeCN | 45 | 81 |
| 5 | DIPEA | THF | 45 | 12 |
| 6 | DIPEA | DMF | 45 | 23 |
| 7 | DIPEA | CH2Cl2 | 45 | 7 |
| 8 | DIPEA | MeCN | 45 | 76 c |
| 9 | DIPEA | MeCN | 25 | 68 |
| 10 | DIPEA | MeCN | 65 | 55 |
| 11 | DIPEA | MeCN | 45 | 79 d |
| 12 | DIPEA | MeCN | 45 | 71 e |
| 13 | DIPEA | MeCN | 45 | 81 f |
| 14 | DIPEA | MeCN | 45 | 70 g |
a Conditions: 1a (0.5 mmol), HCCP (1.1 equiv.), base (5.0 equiv.), solvent (5 mL), rt, activation time (1 h), then thiophenol (6.0 equiv.), 45 °C, 23 h; b Isolated yield; c HCCP (1.0 equiv.); d Thiophenol (5.0 equiv.); e Thiophenol (4.0 equiv.); f DIPEA (6.0 equiv.); g DIPEA (4.0 equiv.).
