Table 3.
HCCP-mediated formation of quinazoline ethers from quinazolin-4(3H)-ones a. 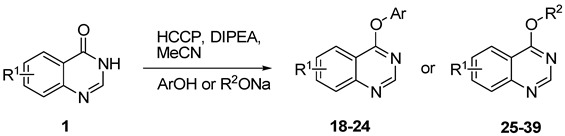
| Entry | Quinazolin-4(3H)-one | ArOH or RONa | Product | Yield (%) b | |
|---|---|---|---|---|---|
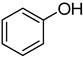
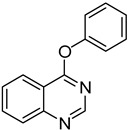
| 1 | 1a |
|
|
18 | 75 |
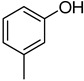
| 2 | 1a |
|
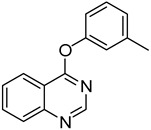
|
19 | 52 |
| 3 | 1a |
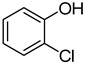
|
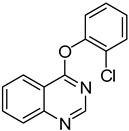
|
20 | 73 |
| 4 | 1a |
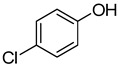
|
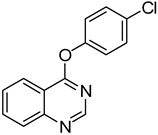
|
21 | 51 |
| 5 | 1b |
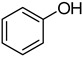
|
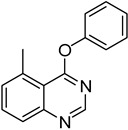
|
22 | 53 c |
| 6 | 1f |
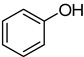
|
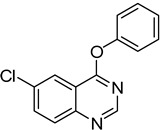
|
23 | 70 |
| 7 | 1g |
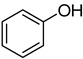
|
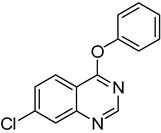
|
24 | 70 |
| 8 | 1a | CH3CH2ONa |
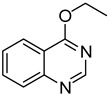
|
25 | 54 |
| 9 | 1b | CH3CH2ONa |
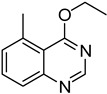
|
26 | 33 c |
| 10 | 1d | CH3CH2ONa |
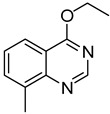
|
27 | 67 |
| 11 | 1g | CH3CH2ONa |
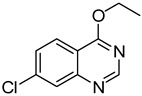
|
28 | 64 |
| 12 | 1a | CH3CH2CH2ONa |
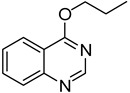
|
29 | 48 |
a Reagents and Conditions: 1 (0.5 mmol), HCCP (1.1 equiv.), DIPEA (5.0 equiv.), MeCN (5 mL), rt, activation time (1 h), then phenols (5.0 equiv.), 45 °C, 23 h; or R2ONa (5.0 equiv.), 45 °C, 3 h; b Isolated yield; c Activation time (20 h).
