Abstract
Co-application of certain types of compounds to conventional antimicrobial drugs can enhance the efficacy of the drugs through a process termed chemosensitization. We show that kojic acid (KA), a natural pyrone, is a potent chemosensitizing agent of complex III inhibitors disrupting the mitochondrial respiratory chain in fungi. Addition of KA greatly lowered the minimum inhibitory concentrations of complex III inhibitors tested against certain filamentous fungi. Efficacy of KA synergism in decreasing order was pyraclostrobin > kresoxim-methyl > antimycin A. KA was also found to be a chemosensitizer of cells to hydrogen peroxide (H2O2), tested as a mimic of reactive oxygen species involved in host defense during infection, against several human fungal pathogens and Penicillium strains infecting crops. In comparison, KA-mediated chemosensitization to complex III inhibitors/H2O2 was undetectable in other types of fungi, including Aspergillus flavus, A. parasiticus, and P. griseofulvum, among others. Of note, KA was found to function as an antioxidant, but not as an antifungal chemosensitizer in yeasts. In summary, KA could serve as an antifungal chemosensitizer to complex III inhibitors or H2O2 against selected human pathogens or Penicillium species. KA-mediated chemosensitization to H2O2 seemed specific for filamentous fungi. Thus, results indicate strain- and/or drug-specificity exist during KA chemosensitization.
Keywords: kojic acid, Aspergillus, Penicillium, Acremonium, Scedosporium, yeast, hydrogen peroxide, mitochondrial respiration inhibitors, chemosensitization
1. Introduction
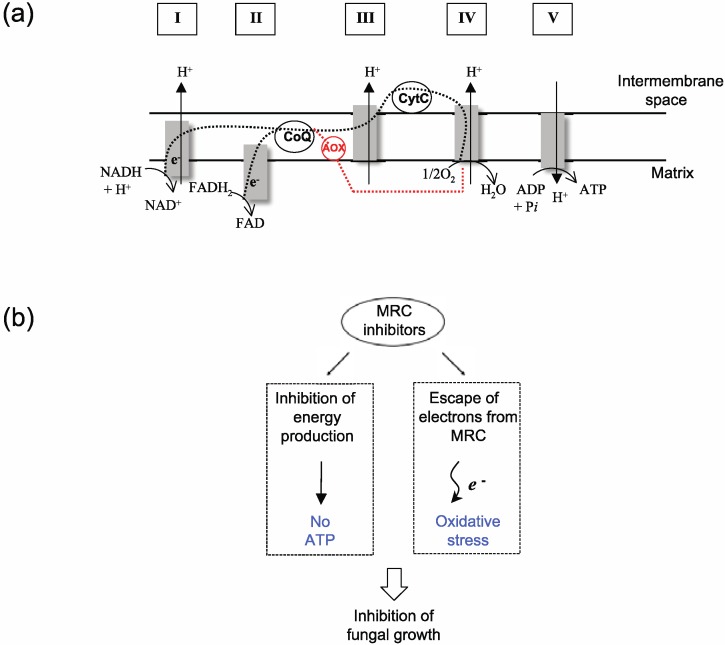
The mitochondrial respiratory chain (MRC) can serve as a valuable molecular target for control of fungal pathogens (Figure 1a). Chemical inhibitors of MRC, such as antimycin A (AntA) or strobilurins (e.g., Pyraclostrobin (PCS), Kresoxim-methyl (Kre-Me), mucidin, etc.), interfere with cellular energy (e.g., ATP) production in fungi [1,2], weakening fungal viability. Coinciding with this interference is an abnormal leakage of electrons from MRC. The escaped electrons can cause oxidative damage to vital components in fungal cells, such as chromosomes, lipid membranes and proteins, resulting in apoptosis or necrosis [1,2] (see Figure 1b for scheme). The antioxidant system in fungi, e.g., glutaredoxins, cytosolic or mitochondrial superoxide dismutases (Cu, Zn- or Mn-SOD), glutathione reductase, plays a protective role in such cases, maintaining cellular homeostasis/integrity from toxic oxidative species [3,4]. Fungi can also overcome the toxicity of MRC inhibitors by expressing alternative oxidase (AOX) (Figure 1a), rendering the completion of electron flow via MRC [5,6]. AOX is insensitive to MRC inhibitors [5,6].
Figure 1.
MRC as a target for control of fungal pathogens. (a) Schematic representation of MRC (Adapted from [2] and [7]). CoQ, Coenzyme Q; CytC, Cytochrome C; e−, Electrons; AOX, Alternative oxidase; Dashed lines (black), Normal route for electron flow; Dashed lines (red), Alternative route for electron flow; I to V, components/complexes of MRC. (b) Mechanism of antifungal action of MRC inhibitors.
With respect to other targets of conventional antifungal drugs already identified (e.g., cell wall/membrane integrity pathway, cell division, signal transduction, and macromolecular synthesis, etc.) [8], MRC is a relatively unexploited target in human fungal pathogens. However, the MRC has been actively used as a drug target for control of malarial parasites, e.g., Plasmodium. For example, the antimalarial drug atovaquone disrupts the mitochondrial electron transport as well as the inner mitochondrial membrane potential (ΔΨm) in parasites [9]. Atovaquone is also used to treat fungal infections such as Pneumocystis jirovecii (pneumonia) [10].
Co-application of certain types of compounds with commercial antimicrobial drugs can increase the effectiveness of drugs through a mechanism termed “chemosensitization” [11,12,13,14]. For example, a prior study showed that the 4-methoxy-2,3,6-trimethylbenzensulfonyl-substituted D-octapeptide chemosensitized cells to the antifungal drug fluconazole (FLC), countering FLC resistance of clinical isolates of Candida pathogens, and of strains of the model yeast Saccharomyces cerevisiae overexpressing multidrug efflux pumps/drug transporter or a lanosterol 14α-demethylase (Erg11p, molecular target of FLC) [11]. Similarly, in bacterial pathogens, application of sub-inhibitory concentrations of squalamine enhanced the antibiotic susceptibility of various Gram-negative bacteria, in both antibiotic-resistant and susceptible strains [12]. Squalamine is thought to modify membrane integrity by increasing permeability of drugs [12].
Meanwhile, co-application of proguanil, which modulates mitochondria in protozoan parasites, resulted in an increased antimalarial activity of atovaquone [15]. Of note is that proguanil-based chemosensitization was specific for atovaquone, i.e., proguanil did not enhance the activities of other MRC inhibitors, such as myxothiazole or AntA [15]. Results indicate “drug-chemosensitizer specificity” exists in the process. Collectively, these studies showed that chemosensitization could ultimately lead to lowering dosages of conventional drugs necessary for effective control of pathogens. It would also lead to preventing development of pathogen resistance to conventional drugs [16].
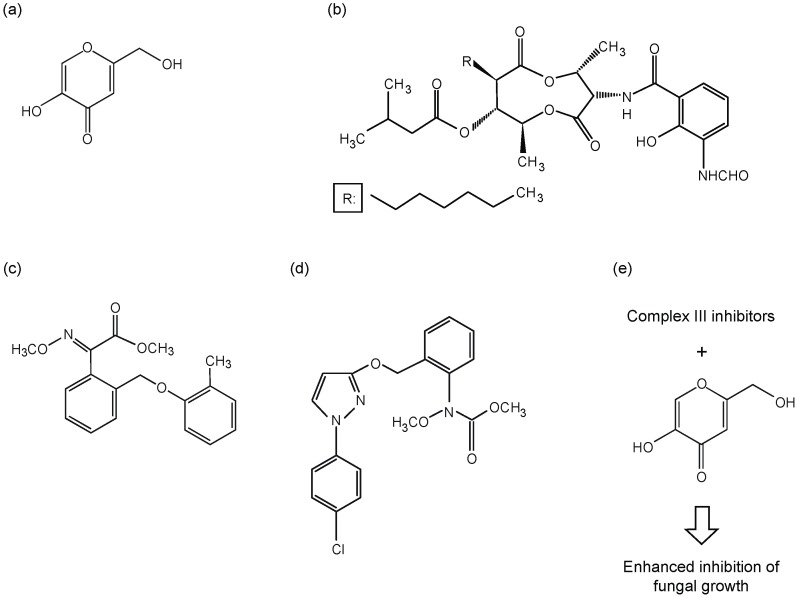
Kojic acid (KA, Figure 2a) is a natural product of some filamentous fungi, mainly certain species of Aspergillus or Penicillium. KA is widely used as a depigmenting agent due to its ability to inhibit the activity of tyrosinase, a key enzyme responsible for melanogenesis in melanoma and melanocytes [17,18,19,20]. From a clinical perspective, KA can potentially inhibit pathogen infection since: (1) it enhances host immunity by stimulating phagocytosis, generating reactive oxygen species (ROS) in macrophages, and potentiating phytohemagglutinin-based proliferation of lymphocytes [21,22]; (2) KA or its structural derivatives directly exert antimicrobial activity against fungal/bacterial pathogens [23]. For instance, KA functions as an antifungal agent against Cryptococcus neoformans (cryptococcosis), where KA also inhibits melanin synthesis necessary for fungal infectivity [24].
Figure 2.
Structures of antifungal compounds examined in this study. (a) KA, (b) AntA, (c) Kre-Me, and (d) PCS; (e) Scheme for enhancement of antifungal activities of complex III inhibitors by KA-mediated chemosensitization.
We previously showed that KA could act as a chemosensitizing agent when co-applied with the polyene antifungal drug amphotericin B (AMB) or hydrogen peroxide (H2O2) against various filamentous fungal or yeast pathogens [25]. The mechanism of antifungal chemosensitization appeared to be modulation of the function of the antioxidant system in the fungus. Noteworthy is that the degree/efficacy of KA-mediated antifungal chemosensitization was related to the kinds of fungal strain and/or drug examined [25]. This tendency is similar to the “drug-chemosensitizer specificity” found in atovaquone-mediated chemosensitization (see above).
In this study, we further investigated if KA, as a chemosensitizer, could improve the activities of complex III inhibitors of MRC (i.e., AntA, Kre-Me, PCS; see Figure 2b–d for structures and 2e for scheme), and thus, possess potential as an active pharmaceutical/agroceutical ingredient, against various filamentous fungi. We included a number of human and plant pathogens, as well as model fungal strains, in our tests (see Table 1; Figure 2e). We observed that human fungal pathogens, i.e., Aspergillus fumigatus, A. terreus, Acremonium sp., and Scedosporium sp., were the most sensitive strains to KA-mediated chemosensitization to complex III inhibitors.
Table 1.
Fungal strains used in this study.
| Fungal strains | Strain characteristics | Source/Reference | |
|---|---|---|---|
| Aspergillus (Human pathogens) | |||
| A. fumigatus MYA-3626 | Aspergillosis, Reference clinical strain | ATCC a | |
| A. fumigatus AF293 | Aspergillosis, Reference clinical strain | SCVMC b | |
| A. fumigatus AF10 | Aspergillosis, Reference clinical strain | SCVMC b | |
| A. fumigatus 94-46 | Aspergillosis, Clinical isolate | SCVMC b | |
| A. fumigatus 92-245 | Aspergillosis, Clinical isolate | SCVMC b | |
| A. terreus UAB673 | Aspergillosis, Clinical isolate | CDC c | |
| A. terreus UAB680 | Aspergillosis, Clinical isolate | CDC c | |
| A. terreus UAB698 | Aspergillosis, Clinical isolate | CDC c | |
| Other filamentous fungi (Human pathogens) | |||
| Acremonium sp. CIMR 95-103 | Clinical isolate | SCVMC b | |
| Scedosporium sp. CIMR 09-246 | Clinical isolate | SCVMC b | |
| Aspergillus (Plant pathogens, etc.) | |||
| A. flavus 4212 g | Kojic acid producer, Plant pathogen, Human pathogen (aspergillosis) | NRRL d | |
| A. parasiticus 2999 | Kojic acid producer, Plant pathogen | NRRL d | |
| A. oryzae A815 | Research strain (model) | FGSC e | |
| A. niger 326 | Plant pathogen | NRRL d | |
| A. ochraceous 5175 | Plant pathogen | NRRL d | |
| A. nidulans A4 | Research strain (model) | FGSC e | |
| Penicillium (Plant pathogens, etc.) | |||
| P. expansum 974 | Plant pathogen | NRRL d | |
| P. expansum W1 | Plant pathogen | [ 26] | |
| P. expansum FR2 | Plant pathogen, Fludioxonil resistant (FLUDR) mutant derived from P. expansum W1 | [ 26] | |
| P. expansum W2 | Plant pathogen | [ 26] | |
| P. expansum FR3 | Plant pathogen, FLUDR mutant derived from P. expansum W2 | [ 26] | |
| P. chrysogenum 824 | Fleming’s penicillin-producing strain | NRRL d | |
| P. griseofulvum 2159 | Plant pathogen | NRRL d | |
| P. griseofulvum 2300 | Plant pathogen | NRRL d | |
| P. digitatum 786 | Plant pathogen | NRRL d | |
| P. italicum 983 | Plant pathogen | NRRL d | |
| P. glabrum 766 | Plant pathogen | NRRL d | |
| Yeasts | |||
| Saccharomyces cerevisiae BY4741 | Model yeast, Parental strain (Mat a his3∆1 leu2∆0 met15∆0 ura3∆0) | SGD f | |
| S. cerevisiae yap1 | Transcription factor Yap1p mutant derived from BY4741 | SGD f | |
| S. cerevisiae sod2 | Mitochondrial superoxide dismutase (Mn-SOD) mutant derived from BY4741 | SGD f | |
| S. cerevisiae sod1 | Cytosolic superoxide dismutase (Cu,Zn-SOD) mutant derived from BY4741 | SGD f | |
| S. cerevisiae glr1 | Glutathione reductase (Glr1p) mutant derived from BY4741 | SGD f | |
a ATCC, American Type Culture Collection, Manassas, VA, USA. b SCVMC, Santa Clara Valley Medical Center, San Jose, CA, USA. c CDC, Centers for Disease Control and Prevention, Atlanta, GA, USA. d NRRL, National Center for Agricultural Utilization and Research, USDA-ARS, Peoria, IL, USA. e FGSC, Fungal Genetics Stock Center, Kansas City, MO, USA. f SGD, Saccharomyces cerevisiae Genome Database [27]. gA. flavus infects both plants and humans.
2. Results and Discussion
2.1. Enhancing Antifungal Activity of H2O2 or Complex III Inhibitors with KA Against Aspergillus or Penicillium Strains: Agar Plate Bioassay
Hydrogen peroxide acts similarly to host-derived ROS, as a host defense response against infecting pathogens. For example, patients with chronic granulomatous disease (CGD) experience high susceptibility to invasive infections by Aspergillus [28]. The phagocytic immune cells of CGD patients cannot induce an oxidative burst because they lack NADPH oxidase, necessary to generate superoxides, the precursor to the antimicrobial ROS H2O2 [28]. Although the infecting fungi rely on their cellular antioxidant system for protection from host ROS, application of KA further enhances host immunity by stimulating phagocytosis and generation of ROS in macrophages (see Introduction) [21,22].
We previously examined KA-mediated chemosensitization to H2O2 and AMB [25]. Besides disrupting fungal plasma membranes, AMB also induces fungal oxidative damage [29,30,31,32] by stimulating ROS production [33]. Thus, we surmised that the effect of KA + AMB would be similar to KA + H2O2. However, unlike with KA + AMB, chemosensitization did not occur with KA + H2O2 in any of the yeast pathogens tested. We concluded that the effectiveness of KA-mediated chemosensitization was fungal strain- and/or drug-specific [25].
Since complex III inhibitors, like AMB, also trigger cellular oxidative stress in fungi (see Introduction), we also compared the effect of KA + complex III inhibitors with that of KA + H2O2 in this study.
2.1.1. Filamentous Fungi
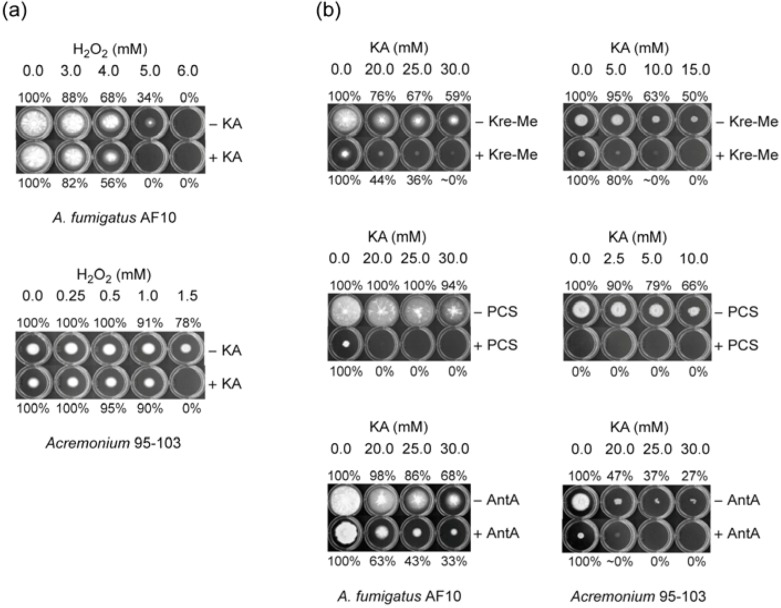
Our initial agar bioassays were performed with the human pathogenic fungi. Co-application of KA (5 mM) with H2O2 (Test concentrations: 0.0, 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 mM) resulted in increased antifungal activities of both compounds, compared to independent treatment of either KA or H2O2, alone (Figure 3a; Table 2). For example, co-application of H2O2 and KA at 5 mM, each, completely inhibited the growth of A. fumigatus AF10 (i.e., no visible germination on plates), whereas independent application of either H2O2 or KA, alone, did not achieve this level of antifungal activity. A similar level of chemosensitization was also observed in other fungi tested, i.e., A. terreus, Acremonium, and Scedosporium, by KA + H2O2 [Figure 3(a); see Table 2 for summary].
Figure 3.
Exemplary agar (PDA) bioassays showing KA-mediated chemosensitization with (a) H2O2 or (b) complex III inhibitors, tested against A. fumigatus AF10 or Acremonium 95-103 (Note: No germination of Acremonium by PCS alone, reflecting hypersensitivity).
Table 2.
Summary of responses of filamentous fungi to KA-mediated chemosensitization with H2O2 or complex III inhibitors (agar plate bioassay) a.
| Strains | H2O2 | Kre-Me | PCS | AntA |
|---|---|---|---|---|
| Human pathogens | ||||
| A. fumigatus MYA-3626 | ++ | + | ++ | + |
| A. fumigatus AF293 | + b | + | ++ | + |
| A. fumigatus AF10 | ++ | + | ++ | + |
| A. fumigatus 94-46 | + | + | ++ | + |
| A. fumigatus 92-245 | + | + | ++ | + |
| A. terreus UAB673 | ++ b | + | ++ | - |
| A. terreus UAB680 | + b | + | ++ | - |
| A. terreus UAB698 | ++ b | - | - | - |
| Acremonium sp. 95-103 | ++ | ++ | n/t c | ++ |
| Scedosporium sp. 09-246 | + | ++ | n/t c | ++ |
| Penicillium strains | ||||
| P. expansum 974 | ++ | - | - | - |
| P. expansum W1 | + | - | - | - |
| P. expansum FR2 | + | - | ++ | - |
| P. expansum W2 | ++ | - | - | - |
| P. expansum FR3 | ++ | - | + | - |
| P. chrysogenum 824 | ++ | - | - | - |
| P. griseofulvum 2159 | ++ | - | - | - |
| P. griseofulvum 2300 | - | - | - | - |
| P. digitatum 786 | ++ | n/t d | n/t c | + |
| P. italicum 983 | - | + | ++ | - |
| P. glabrum 766 | - | + | + | + |
| Other Aspergillus strains | ||||
| A. flavus 4212 | - | - | - | - |
| A. parasiticus 2999 | - | - | - | - |
| A. oryzae A815 | - | - | - | - |
| A. niger 326 | - | - | - | - |
| A. ochraceous 5175 | - | - | - | - |
| A. nidulans A4 | - | + | + | - |
a +, Enhancement of antifungal activity after co-application (reduced radial growth of fungi); ++, Enhancement of antifungal activity after co-application (no germination of fungi); -, No enhancement of antifungal activity after co-application. b [25]. c n/t, Not tested due to no growth of fungi w/ PCS (25 μM) alone (i.e., hypersensitivity to PCS alone). d n/t, Not tested due to no growth of fungi w/ Kre-Me (25 μM) alone (i.e., hypersensitivity to Kre-Me alone).
Next we found that KA-mediated chemosensitization could also be achieved with complex III inhibitors in most of the human pathogens tested (Figure 3b; Table 2). KA (Test concentrations: 0.0, 2.5, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0 mM) was co-applied with 25 μM of complex III inhibitors (i.e., PCS, Kre-Me or AntA) in agar bioassays. For example, co-application of KA (20 mM or above) and PCS (25 μM) completely inhibited the growth of A. fumigatus AF10 (i.e., no visible germination on plates). Whereas, independent application of KA or PCS, alone, did not result in such a level of antifungal activity. Levels of enhancement of antifungal activity also depended upon types of complex III inhibitors co-applied. PCS exerted the highest activity, followed by Kre-Me and AntA. Similar trends were also observed in other pathogens, such as A. terreus, Acremonium and Scedosporium(Figure 3a; see Table 2 for summary). The only exceptions were A. terreus UAB698 (no enhancement of sensitivity by KA + any of the complex III inhibitors) and A. terreus UAB673/680 (no enhancement of sensitivity by KA + AntA), respectively. Therefore, sensitivity of fungal strains to KA-mediated chemosensitization with complex III inhibitors ranged, from highest to lowest, as follows: Acremonium, Scedosporium > A. fumigatus > A. terreus. Of note is that, although human pathogens were also sensitive to KA + H2O2, levels/degrees of their sensitivity were generally not parallel to that of KA + complex III inhibitors (see Table 2).
Agar bioassays performed on Penicillium strains, mostly plant pathogens, showed that co-application of KA with H2O2 resulted in enhancement of antifungal activities of both compounds (KA and H2O2), except P. griseofulvum 2300, P. italicum and P. glabrum, which were insensitive to this chemosensitization (Table 2; Figure data not shown).
KA-mediated chemosensitization was also performed using the complex III inhibitors on the Penicillium strains. Unlike the human pathogens tested, chemosensitization was more limited with the Penicillium strains, being effective only in strains, P. expansum FR2 and FR3 (both being fludioxonil (FLUD) resistant strains), P. digitatum, P. italicum and P. glabrum with KA + PCS (Table 2; PCS was the most effective complex III inhibitor in this test). Levels of strain sensitivity in decreasing order with KA + PCS were: P. digitatum > P. italicum, P. expansum FR2 > P. glabrum, P. expansum FR3. P. digitatum, P. italicum, and P. glabrum were also sensitive to KA + Kre-Me or AntA. However, Penicillium strains were generally not as sensitive to KA-mediated chemosensitization with complex III inhibitors as human pathogens. As observed in human pathogens, levels/degrees of fungal sensitivity to KA + H2O2 were not parallel to that of KA + complex III inhibitors (see Table 2).
Agar bioassays were performed on six other strains of Aspergillus, mainly plant pathogens or model strains (A. flavus: pathogenic to both plants and humans). These assays showed that co-application of KA with H2O2 or complex III inhibitors resulted in no enhancement of antifungal activity of any compound tested (KA, H2O2 or complex III inhibitors), except A. nidulans, which showed sensitivity to KA + PCS or Kre-Me (Table 2; Figure data not shown).
In summary, our results with filamentous fungi show that KA-mediated chemosensitization is fungal strain- or drug (compound)-dependent. Strain sensitivity to KA + complex III inhibitors and/or H2O2 varied as follows (in decreasing order): human pathogens (A. fumigatus, A. terreus, Acremonium sp., Scedosporium sp.; Mostly sensitive to KA + complex III inhibitors and KA + H2O2) > Penicillium species (Certain strains were sensitive to KA + complex III inhibitors, while many strains were sensitive to H2O2) > all other Aspergillus species (A. flavus, A. parasiticus, A. oryzae, A. niger, A. ochraceous, A. nidulans; Only A. nidulans was sensitive to KA +PCS or KA + Kre-Me. No strain was sensitive to KA + H2O2).
2.1.2. Antioxidant, but Not Antifungal, Effect of KA in Wild Type and Antioxidant Mutants of the Model Yeast Saccharomyces cerevisiae
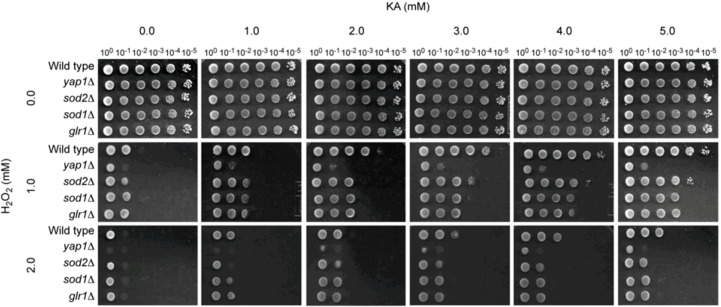
In our previous study, KA-mediated chemosensitization with H2O2 was not effective in any of the yeast pathogens tested [25]. Therefore, in the present study, we attempted to examine how the treatment of KA + H2O2 was related to various functions of antioxidant system of yeasts using S. cerevisiae as a model. For this study, we used a yeast dilution bioassay (see Experimental Section) and tested a wild type and four antioxidant mutant (gene knock-out) strains of S. cerevisiae as follows: (1) yap1Δ (Yap1p, a transcription factor, regulates the expression of four downstream genes within the antioxidant system, i.e., GLR1 (glutathione reductase), YCF1 (a glutathione S-conjugate pump), TRX2 (thioredoxin), and GSH1 (γ-glutamylcysteine synthetase [34,35]); (2) sod1Δ (Cu,Zn-SOD); (3) sod2Δ (Mn-SOD); and (4) glr1Δ (Glr1p, glutathione reductase; see Saccharomyces Genome Database [27]). These representative mutants play key roles in maintaining cellular redox homeostasis in both enzymatic (e.g., ROS radical-scavenging) and non-enzymatic (e.g., glutathione homeostasis) aspects. Worth noting is that S. cerevisiae has also been developed as a model system for studying atovaquone resistance [36].
To our surprise, in these yeast strains, KA mainly acted as an antioxidant, but not as an antifungal chemosensitizer (Figure 4). For example, when wild type or mutants were treated with 1 mM of H2O2 alone, all yeast strains showed sensitive responses, as reflected in no growth of cells at 10−2 to 10−5 dilution spots (Figure 4). As expected, yap1Δ, which regulates the expression of four downstream genes in the antioxidant system, was more sensitive to H2O2 (i.e., no growth at 10−1 dilution spot) than any other yeast strains. However, as shown in Figure 4, co-application of KA with H2O2 ameliorated the H2O2-triggered oxidative stress, resulting in enhancement of the growth of all yeasts tested. For example, the wild type showed growth recovery up to 100,000-fold dilution (the 10−5 dilution spot), revealing this strain fully recovered from oxidative stress induced by H2O2 when KA was co-applied. Additionally, the sod1Δ, sod2Δ and glr1Δ mutants grew up to 10−3 to 10−4 dilution spots and yap1Δ grew up to 10−1 dilution spot when H2O2 was co-applied with 5 mM KA.
Figure 4.
Agar (SG)-based yeast-cell dilution bioassay showing antioxidant effect of KA to H2O2-treated S. cerevisiae strains (100 to 10−5: yeast dilution rates).
The antioxidant capacity of KA was also commensurate with KA concentrations. Although yeast strains showed increased sensitivity to 2 mM of H2O2, similar trends of antioxidation activity by KA were also observed (Figure 4). Thus, overall, the results indicate KA has a different effect, depending on types of fungi examined. That is, KA functions as an antioxidant in S. cerevisiae, while it acts as an antifungal chemosensitizer in certain of the filamentous fungi tested. KA may induce different transcriptional programs in S. cerevisiae than in filamentous fungi. Further studies, such as genome-wide gene expression profiling, are warranted to determine the precise mechanism of antioxidation in and/or insensitivity of yeast to KA + H2O2 chemosensitization.
2.2. Calculating Levels of Compound Interactions by Using Microtiter Plate (Microdilution) Bioassays: Human Pathogens, Penicillium Strains or A. nidulans
Based on results from the agar bioassay on filamentous fungi (shown above), levels of compound interactions between KA and PCS (the most potent complex III inhibitor according to this study) were assessed only for the strains sensitive to KA-mediated chemosensitization (i.e., most human pathogens, five Penicillium strains, and A. nidulans) using triplicate, microtiter-plate checkerboard bioassays (Clinical Laboratory Standards Institute (CLSI) M38-A) [37] with concentration ranges of KA (0, 1, 2, 4, 8, 16, 32, 64 mM) and PCS (0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 µg/mL) (see Experimental Section). The effect of KA + Kre-Me (0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 µg/mL) was also determined only for Acremonium, Scedosporium and P. digitatum, which were the most sensitive strains to complex III inhibitors (see Table 2).
2.2.1. Co-Application of KA and PCS
Synergistic Fractional Inhibitory Concentration Indices (FICIs; see Experimental Section for calculations) were found between KA and PCS for most human pathogens (A. fumigatus, A. terreus, Acremonium sp., Scedosporium sp.) and A. nidulans (Table 3). Despite the absence of calculated “synergism”, as determined by “indifferent” interactions [38] (Table 3), there was enhanced antifungal activity of KA and PCS (i.e., chemosensitization) in Acremonium, which was reflected in lowered Minimum Inhibitory Concentrations (MICs) of each compound when combined. However, synergistic Fractional Fungicidal Concentration Indices (FFCIs) (at the level of ≥ 99.9% fungal death) between KA and PCS occurred only in Acremonium (Table 3), indicating the KA-mediated chemosensitization with PCS is fungistatic, not fungicidal, in most strains tested.
Table 3.
Antifungal chemosensitization of KA (mM) to PCS (μg/mL) tested against filamentous fungi: summary of CLSI-based microdilution bioassays a.
| Strains (Human pathogens and A. nidulans) | Compounds | MIC alone | MIC combined | FICI |
| A. fumigatus AF293 | KA | 64 | 16 | 0.3 |
| PCS | >16 b | 1 | ||
| A. fumigatus MYA-3626 | KA | >64 c | 16 | 0.4 |
| PCS | >16 | 8 | ||
| A. fumigatus AF10 | KA | 64 | 16 | 0.4 |
| PCS | >16 | 4 | ||
| A. fumigatus 92-245 | KA | >64 | 16 | 0.4 |
| PCS | >16 | 8 | ||
| A. fumigatus 94-46 | KA | >64 | 16 | 0.4 |
| PCS | >16 | 8 | ||
| A. terreus UAB673 | KA | 64 | 8 | 0.1 |
| PCS | >16 | 0.5 | ||
| A. terreus UAB 680 | KA | 64 | 8 | 0.2 |
| PCS | >16 | 1 | ||
| A. nidulans A4 | KA | >64 | 32 | 0.5 |
| PCS | >16 | 8 | ||
| Acremonium sp. 95-103 | KA | 64 | 16 | 0.8 |
| PCS | 0.25 | 0.125 | ||
| Scedosporium sp. 09-246 | KA | 64 | 4 | 0.2 |
| PCS | 1 | 0.125 | ||
| Mean | KA | 89.6 | 14.8 | 0.3 |
| PCS | 25.7 | 3.9 | ||
| t-test d | KA | - | p < 0.005 | - |
| PCS | - | p < 0.005 | - | |
| Strains (Human pathogens and A. nidulans) | Compounds | MFC alone | MFC combined | FFCI |
| Acremonium sp. 95-103 | KA | >64 | 32 | 0.5 |
| PCS | 2 | 0.5 | ||
| All other strains | KA | >64 | >64 | 2 |
| PCS | >16 | >16 | ||
| Mean | KA | 128 | 118.4 | 1.9 |
| PCS | 29 | 28.9 | ||
| t-test | KA | - | p < 0.5 | - |
| PCS | - | p < 1.0 | - | |
| Strains (Penicillium strains) | Compounds | MIC alone | MIC combined | FICI |
| P. expansum FR2 | KA | >64 | 16 | 0.4 |
| PCS | 2 | 0.5 | ||
| P. expansum FR3 | KA | >64 | 32 | 0.8 |
| PCS | 2 | 1 | ||
| P. glabrum 766 | KA | >64 | 32 | 0.4 |
| PCS | >16 | 4 | ||
| P. digitatum 786 | KA | >64 | 2 | 0.5 |
| PCS | 0.25 | 0.125 | ||
| P. italicum 983 | KA | >64 | 16 | 0.3 |
| PCS | >16 | 4 | ||
| Mean | KA | 128 | 19.6 | 0.3 |
| PCS | 13.7 | 1.9 | ||
| t-test | KA | - | p < 0.005 | - |
| PCS | - | p < 0.5 | - | |
| Strains (Penicillium strains) | Compounds | MFC alone | MFC combined | FFCI |
| P. glabrum 766 | KA | >64 | 64 | 1 |
| PCS | >16 | 16 | (99.8%) | |
| P. italicum 983 | KA | >64 | 64 | 1 |
| PCS | >16 | 16 | (99.8%) | |
| All other strains | KA | >64 | > 64 | 2 |
| PCS | >16 | >16 | ||
| Mean | KA | 128 | 102.4 | 1.6 |
| PCS | 32 | 25.6 | ||
| t-test | KA | - | p < 0.5 | - |
| PCS | - | p < 0.5 | - |
a MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration, FICI: Fractional Inhibitory Concentration Indices, FFCI: Fractional Fungicidal Concentration Indices. Synergistic FICIs and FFCI were in bold. b PCS was tested up to 16 μg/mL. For calculation purpose, 32 μg/mL (doubling of 16 μg/mL) was used. c KA was tested up to 64 mM. For calculation purpose, 128 mM (doubling of 64 mM) was used. d Student’s t-test for paired data (combined, i.e., chemosensitization) was vs. mean MIC or MFC of each compound (alone, i.e., no chemosensitization) determined in strains (Calculation was based on [39]).
Synergistic FICIs between KA and PCS also occurred in four Penicillium strains (Table 3). Despite the absence of calculated “synergism” [38] (Table 3), there was enhanced antifungal activity of KA and PCS (i.e., chemosensitization) also in P. expansum FR3 (FLUD resistant strain), which was reflected in lowered MICs of each compound when combined. However, synergistic FFCI (at the level of ≥99.9% fungal death) between KA and PCS was not achieved in any of the Penicillium strains examined (Table 3), indicating that, as in the human pathogens/A. nidulans (See above), the KA-mediated chemosensitization with PCS is mostly fungistatic, not fungicidal, in Penicillium strains (Lowered Minimum Fungicidal Concentrations (MFCs), although not “synergistic” level, were observed in P. glabrum and P. italicum at the level of ≥99.8% fungal death; see Table 3).
2.2.2. Strains Hypersensitive to Complex III Inhibitors: Testing Acremonium, Scedosporium, P. digitatum with Kre-Me
KA + Kre-Me was also examined in Acremonium, Scedosporium and P. digitatum, which were the most sensitive strains to complex III inhibitors (see Table 2). We tried to determine the level of sensitivity of these strains to Kre-Me, which is less potent than PCS (see Figure 3 and Table 2). Consistently, synergistic FICIs between KA and Kre-Me occurred in all strains tested (Table 4). However, synergistic FFCIs (at the level of ≥99.9% fungal death) between KA and Kre-Me were not achieved in any of the strains examined (Table 4), while lowered MFCs for both KA and Kre-Me were observed in Acremonium (FFCI = 0.6). Acremonium sp. is the only strain with low FFCI values for both PCS and Kre-Me, i.e., 0.5PCS and 0.6Kre-Me, respectively (see Table 3, Table 4). Results further confirmed the sensitive responses of Acremonium, Scedosporium and P. digitatum to complex III inhibitors (both PCS and Kre-Me).
Table 4.
Antifungal chemosensitization of KA (mM) to Kre-Me (μg/mL) tested against Acremonium, Scedosporium or P. digitatum strains: summary of CLSI-based microdilution bioassays a.
| Strains | Compounds | MIC alone | MIC combined | FICI |
| Acremonium sp. 95-103 | KA | 64 | 8 | 0.2 |
| Kre-Me | 16 | 1 | ||
| Scedosporium sp. 09-246 | KA | 64 | 8 | 0.2 |
| Kre-Me | >16 b | 1 | ||
| P. digitatum 786 | KA | >64 c | 8 | 0.1 |
| Kre-Me | 8 | 0.5 | ||
| Mean | KA | 85.3 | 8 | 0.1 |
| Kre-Me | 18.7 | 0.8 | ||
| t-test d | KA | - | p < 0.05 | - |
| Kre-Me | - | p < 0.1 | - | |
| Strains | Compounds | MFC alone | MFC combined | FFCI |
| Acremonium sp. 95-103 | KA | >64 | 64 | 0.6 |
| Kre-Me | >16 | 2 | ||
| Scedosporium sp. 09-246 & P. digitatum 786 | KA | >64 | >64 | 2 |
| Kre-Me | >16 | >16 | ||
| Mean | KA | 128 | 106.7 | 1.5 |
| Kre-Me | 32 | 22 | ||
| t-test d | KA | - | p < 0.5 | - |
| Kre-Me | - | p < 0.5 | - |
a MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration, FICI: Fractional Inhibitory Concentration Indices, FFCI: Fractional Fungicidal Concentration Indices. Synergistic FICIs were in bold. b Kre-Me was tested up to 16 μg/mL. For calculation purpose, 32 μg/mL (doubling of 16 μg/mL) was used. c KA was tested up to 64 mM. For calculation purpose, 128 mM (doubling of 64 mM) was used. d Student’s t-test for paired data (combined, i.e., chemosensitization) was vs. mean MIC or MFC of each compound (alone, i.e., no chemosensitization) determined in three strains (Calculation was based on [39]).
The results of all CLSI-based checkerboard (chemosensitization) tests (i.e., KA + PCS or Kre-Me in filamentous fungi) are summarized in Table 5. As shown in the Table, the FICIs for thirteen strains (out of fifteen strains) w/PCS and for three fungi (the most sensitive strains to complex III inhibitors) w/Kre-Me were synergistic. Whereas, FFCI for only Acremonium sp. was synergistic, indicating the KA-mediated chemosensitization with complex III inhibitors exerted mostly fungistatic (but not fungicidal) effects.
Table 5.
Summary of responses of filamentous fungi to the co-application of KA with PCS and/or Kre-Me (CLSI-based microdilution bioassays).
| Fungal strains | Agents co-applied | |
|---|---|---|
| PCS (FICI, FFCI) a | Kre-Me (FICI, FFCI) a | |
| Human pathogens | ||
| A. fumigatus AF293 | (0.3, 2.0) | nt b |
| A. fumigatus MYA-3626 | (0.4, 2.0) | nt |
| A. fumigatus AF10 | (0.4, 2.0) | nt |
| A. fumigatus 92-245 | (0.4, 2.0) | nt |
| A. fumigatus 94-46 | (0.4, 2.0) | nt |
| A. terreus UAB673 | (0.1, 2.0) | nt |
| A. terreus UAB680 | (0.2, 2.0) | nt |
| Acremonium sp. 95-103 | (0.8, 0.5) | (0.2, 0.6) |
| Scedosporium sp. 09-246 | (0.2, 2.0) | (0.2, 2.0) |
| Plant pathogens | ||
| P. expansum FR2 | (0.4, 2.0) | nt |
| P. expansum FR3 | (0.8, 2.0) | nt |
| P. glabrum 766 | (0.4, 1.0) | nt |
| P. italicum 983 | (0.3, 1.0) | nt |
| P. digitatum 786 | (0.5, 2.0) | (0.1, 2.0) |
| Other Aspergillus | ||
| A. nidulans A4 | (0.5, 2.0) | nt |
3. Experimental
3.1. Fungal Strains and Culture Conditions
Human pathogens (Aspergillus fumigatus, A. terreus, Acremonium sp., Scedosporium sp.) (see Table 1) were grown at 35 °C on potato dextrose agar (PDA; Sigma, St. Louis, MO, USA). All other filamentous fungi were grown at 28 °C on PDA. Yeast strains (i.e., wild type and gene deletion mutants of Saccharomyces cerevisiae; see Table 1) were cultured on Synthetic Glucose (SG; Yeast nitrogen base without amino acids 0.67%, glucose 2% with appropriate supplements: uracil 0.02 mg/mL, amino acids 0.03 mg/mL) or Yeast Peptone Dextrose (YPD; Bacto yeast extract 1%, Bacto peptone 2%, glucose 2%) medium at 30 °C.
3.2. Chemicals
Antifungal chemosensitizing agent [kojic acid (KA)], antifungal drugs [antimycin A (AntA), kresoxim methyl (Kre-Me), pyraclostrobin (PCS)] and oxidizing agent [hydrogen peroxide (H2O2)] were procured from Sigma Co. Each compound was dissolved in dimethyl sulfoxide (DMSO; absolute DMSO amount: <1% in media), except H2O2, which was dissolved in water, before incorporation into culture media. In all tests, control plates (i.e., “No treatment”) contained DMSO at levels equivalent to that of cohorts receiving antifungal agents, within the same set of experiments.
3.3. Antifungal Bioassay
3.3.1. Agar Plate Bioassay: Filamentous Fungi
In the agar plate bioassay, measurement of sensitivities of filamentous fungi to the antifungal agents was based on percent (%) radial growth of treated compared to control (“No treatment”) fungal colonies (see text for test concentrations.) [40]. Minimum inhibitory concentration (MIC) values on agar plates were determined based on triplicate bioassays, and defined as the lowest concentration of agents where no fungal growth was visible on the plate. For the above assays, fungal conidia (5 × 104 CFU/mL) were diluted in phosphate-buffered saline (PBS) and applied as a drop onto the center of PDA plates with or without antifungal compounds. Growth was observed for three to seven days to determine cellular sensitivities to compounds.
3.3.2. Microtiter Plate (microdilution) Bioassay: Filamentous Fungi
To determine antifungal chemosensitizing activities of KA (0, 1, 2, 4, 8, 16, 32, 64 mM) to complex III inhibitors (PCS or Kre-Me: 0, 0.125, 0.25, 0.5, 1, 2, 4, 8, 16 µg/mL) in filamentous fungi, checkerboard bioassays (0.4 × 104–5 × 104 CFU/mL) were performed in microtiter wells using a broth microdilution method (with RPMI 1640 medium; Sigma Co.), according to those outlined by the Clinical Laboratory Standards Institute (CLSI) M38-A [37]. Minimum inhibitory concentrations (MICs), lowest concentration of agent(s) showing no visible fungal growth, were assessed after 48 and 72 h. Minimum fungicidal concentrations (MFCs), lowest concentration of agents showing ≥ 99.9% fungal death (except where noted in Tables), were determined (following completion of MIC assays) wherein entire volumes of microtiter wells (200 μL) were spread onto individual PDA plates, and cultured for another 48 and 72 h. Compound interactions, Fractional Inhibitory Concentration Indices (FICIs) and Fractional Fungicidal Concentration Indices (FFCI), were calculated as follows: FICI or FFCI = (MIC or MFC of compound A in combination with compound B/MIC or MFC of compound A, alone) + (MIC or MFC of compound B in combination with compound A/MIC or MFC of compound B, alone). Interactions were defined as: “synergistic” (FICI or FFCI ≤ 0.5) or “indifferent” (FICI or FFCI > 0.5–4) [38]. Statistical analysis was based on [39].
3.3.3. Agar Plate Bioassay: S. cerevisiae
Petri plate-based yeast dilution bioassays were performed on the wild type and antioxidant mutants (yap1Δ, sod1Δ, sod2Δ, glr1Δ) to assess effects of KA + H2O2 on the antioxidant system. Yeast strains were exposed to 1 to 5 mM of KA, w/o or w/H2O2 (1 or 2 mM) on SG for 5 to 7 days. These assays were performed in duplicate on SG agar following previously described protocols [41].
4. Conclusions
In this study, KA enhanced antifungal activities of MRC inhibitor(s) or H2O2 as follows: (1) Most human pathogens tested (i.e., A. fumigatus, A. terreus, Acremonium sp., Scedosporium sp.) were sensitive to both KA + complex III inhibitors and KA + H2O2, except A. terreus UAB698 (no chemosensitization w/all complex III inhibitors tested) and A. terreus UAB673/680 (no chemosensitization w/AntA); (2) Most of the plant pathogenic Penicillium species were sensitive to KA + H2O2, except P. griseofulvum 2300, P. italicum and P. glabrum (no chemosensitization); (3) Some Penicillium species (i.e., P. digitatum, P. italicum, P. glabrum, and FLUD-resistant P. expansum FR2/FR3) were sensitive to KA + at least one of the complex III inhibitors. However, all other Penicillium species were insensitive to KA + complex III inhibitors (no chemosensitization); (4) All other Aspergillus species (i.e., A. flavus, A. parasiticus, A. oryzae, A. niger, A. ochraceous, A. nidulans) were insensitive to KA + complex III inhibitors and/or KA + H2O2 (no chemosensitization), except A. nidulans, which was sensitive to KA + PCS or Kre-Me (chemosensitization). Further studies are required to determine the mechanism(s) governing the variability of these Aspergillus strains to KA-mediated chemosensitization; (5) Most compound interactions at MIC level (i.e., FICI) between KA and PCS or Kre-Me, determined by CLSI method, resulted in synergism, except Acremonium sp. (KA + PCS) and P. expansum FR3 (KA + Kre-Me), which resulted in a certain level of positive interaction between compounds, but not synergism; (6) The antifungal chemosensitizing capacity of KA appears to be fungal strain-specific (i.e., specific for certain human pathogens or Penicillium species only) as well as fungal isolate-dependent (i.e., A. terreus). KA mainly functions as an antioxidant in yeasts; and (7) Strain sensitivity to KA + complex III inhibitors or H2O2 varied as follows (in decreasing order): human fungal pathogens > Penicillium species > all other Aspergillus species.
In conclusion, KA, which is a relatively safe, natural compound to humans [42], shows some potential to serve as an antifungal chemosensitizing agent in combination with complex III inhibitors. This potential appears to be greatest with those filamentous fungi tested that are mainly pathogenic to humans. Chemosensitization can lower dosage levels of antifungal drugs necessary for effective control of fungi. Thus, use of safe chemosensitizing agents that selectively debilitate the fungal pathogen may be a viable approach to circumvent potential side-effects commonly associated with antimycotic therapy.
Acknowledgments
We thank Arun Balajee, Centers for Disease Control and Prevention, Atlanta, GA, USA, and David Stevens, Santa Clara Valley Medical Center, San Jose, CA, USA, for providing us the strains of A. terreus and A. fumigatus, respectively. We also thank Chang-Lin Xiao, Department of Plant Pathology, Washington State University, Wenatchee, WA, USA, for providing us the wild type and fludioxonil-resistant P. expansum strains. This research was conducted under USDA-ARS CRIS Project 5325-42000-037-00D.
Footnotes
Sample Availability: Not available.
References
- 1.Fujita K., Tani K., Usuki Y., Tanaka T., Taniguchi M. Growth inhibition dependent on reactive oxygen species generated by C9-UK-2A, a derivative of the antifungal antibiotic UK-2A, in Saccharomyces cerevisiae. J. Antibiot. (Tokyo) 2004;57:511–517. doi: 10.7164/antibiotics.57.511. [DOI] [PubMed] [Google Scholar]
- 2.Ruy F., Vercesi A.E., Kowaltowski A.J. Inhibition of specific electron transport pathways leads to oxidative stress and decreased Candida albicans proliferation. J. Bioenerg. Biomembr. 2006;38:129–135. doi: 10.1007/s10863-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 3.Longo V.D., Gralla E.B., Valentine J.S. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J. Biol. Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 4.Grant C.M. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 2001;39:533–541. doi: 10.1046/j.1365-2958.2001.02283.x. [DOI] [PubMed] [Google Scholar]
- 5.Costa-de-Oliveira S., Sampaio-Marques B., Barbosa M., Ricardo E., Pina-Vaz C., Ludovico P., Rodrigues A.G. An alternative respiratory pathway on Candida krusei: Implications on susceptibility profile and oxidative stress. FEMS Yeast Res. 2012;12:423–429. doi: 10.1111/j.1567-1364.2012.00789.x. [DOI] [PubMed] [Google Scholar]
- 6.Inoue K., Tsurumi T., Ishii H., Park P., Ikeda K. Cytological evaluation of the effect of azoxystrobin and alternative oxidase inhibitors in Botrytis cinerea. FEMS Microbiol. Lett. 2012;326:83–90. doi: 10.1111/j.1574-6968.2011.02438.x. [DOI] [PubMed] [Google Scholar]
- 7.McIntosh L. Molecular biology of the alternative oxidase. Plant Physiol. 1994;105:781–786. doi: 10.1104/pp.105.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrosky-Zeichner L., Casadevall A., Galgiani J.N., Odds F.C., Rex J.H. An insight into the antifungal pipeline: Selected new molecules and beyond. Nat. Rev. Drug Discov. 2010;9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava I.K., Rottenberg H., Vaidya A.B. Atovaquone, a broad spectrum antiparasitic drug, collapses mitochondrial membrane potential in a malarial parasit. J. Biol. Chem. 1997;272:3961–3966. doi: 10.1074/jbc.272.7.3961. [DOI] [PubMed] [Google Scholar]
- 10.MedlinePlus. Atovaquone. [(accessed on 12 December 2012)]. Available online: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a693003.html/
- 11.Niimi K., Harding D.R., Parshot R., King A., Lun D.J., Decottignies A., Niimi M., Lin S., Cannon R.D., Goffeau A., et al. Chemosensitization of fluconazole resistance in Saccharomyces cerevisiae and pathogenic fungi by a D-octapeptide derivative. Antimicrob. Agents Chemother. 2004;48:1256–1271. doi: 10.1128/AAC.48.4.1256-1271.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavigne J.P., Brunel J.M., Chevalier J., Pages J.M. Squalamine, an original chemosensitizer to combat antibiotic-resistant gram-negative bacteria. J. Antimicrob. Chemother. 2010;65:799–801. doi: 10.1093/jac/dkq031. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A.K., Tripathi S.K., Xu T., Jacob M.R., Li X.C., Clark A.M. Exploring the molecular basis of antifungal synergies using genome-wide approaches. Front. Microbiol. 2012;3:115. doi: 10.3389/fmicb.2012.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell B.C., Chan K.L., Kim J.H. Chemosensitization as a means to augment commercial antifungal agents. Front. Microbiol. 2012;3:79. doi: 10.3389/fmicb.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava I.K., Vaidya A.B. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob. Agents Chemother. 1999;43:1334–1339. doi: 10.1128/aac.43.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J.H., Chan K.L., Mahoney N., Campbell B.C. Antifungal activity of redox-active benzaldehydes that target cellular antioxidation. Ann. Clin. Microbiol. Antimicrob. 2011;10:23. doi: 10.1186/1476-0711-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentley R. From miso, sake and shoyu to cosmetics: A century of science for kojic acid. Nat. Prod. Rep. 2006;23:1046–1062. doi: 10.1039/b603758p. [DOI] [PubMed] [Google Scholar]
- 18.Chang T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009;10:2440–2475. doi: 10.3390/ijms10062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyden J.J., Shergill B., Micali G., Downie J., Wallo W. Natural options for the management of hyperpigmentation. J. Eur. Acad. Dermatol. Venereol. 2011;25:1140–1145. doi: 10.1111/j.1468-3083.2011.04130.x. [DOI] [PubMed] [Google Scholar]
- 20.Lajis A.F., Hamid M., Ariff A.B. Depigmenting effect of kojic acid esters in hyperpigmented B16F1 melanoma cells. J. Biomed. Biotechnol. 2012;2012:952452. doi: 10.1155/2012/952452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa Y., Akamatsu H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation. 1991;15:303–315. doi: 10.1007/BF00917315. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues A.P., Carvalho A.S., Santos A.S., Alves C.N., do Nascimento J.L., Silva E.O. Kojic acid, a secondary metabolite from Aspergillus sp., acts as an inducer of macrophage activation. Cell Biol. Int. 2011;35:335–343. doi: 10.1042/CBI20100083. [DOI] [PubMed] [Google Scholar]
- 23.Reddy B.V., Reddy M.R., Madan C., Kumar K.P., Rao M.S. Indium(III) chloride catalyzed three-component coupling reaction: A novel synthesis of 2-substituted aryl(indolyl)kojic acid derivatives as potent antifungal and antibacterial agents. Bioorg. Med. Chem. Lett. 2010;20:7507–7511. doi: 10.1016/j.bmcl.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Chee H.Y., Lee E.H. Fungistatic activity of kojic acid against human pathogenic fungi and inhibition of melanin production in Cryptococcus neoformans. Mycobiology. 2003;31:248–250. doi: 10.4489/MYCO.2003.31.4.248. [DOI] [Google Scholar]
- 25.Kim J.H., Chang P.K., Chan K.L., Faria N.C., Mahoney N., Kim Y.K., Martins Mde L., Campbell B.C. Enhancement of commercial antifungal agents by kojic acid. Int. J. Mol. Sci. 2012;13:13867–13880. doi: 10.3390/ijms131113867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H.X., Xiao C.L. Characterization of fludioxonil-resistant and pyrimethanil-resistant phenotypes of Penicillium expansum from apple. Phytopathology. 2008;98:427–435. doi: 10.1094/PHYTO-98-4-0427. [DOI] [PubMed] [Google Scholar]
- 27.Saccharomyces Genome Database. [(accessed on 8 December 2012)]. Available online: http://www.yeastgenome.org/
- 28.Dinauer M.C. The respiratory burst oxidase and the molecular genetics of chronic granulomatous disease. Crit. Rev. Clin. Lab. Sci. 1993;30:329–369. doi: 10.3109/10408369309082591. [DOI] [PubMed] [Google Scholar]
- 29.Sokol-Anderson M.L., Brajtburg J., Medoff G. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 1986;154:76–83. doi: 10.1093/infdis/154.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Graybill J.R., Burgess D.S., Hardin T.C. Key issues concerning fungistatic versus fungicidal drugs. Eur. J. Clin. Microbiol. Infect. Dis. 1997;16:42–50. doi: 10.1007/BF01575120. [DOI] [PubMed] [Google Scholar]
- 31.An M., Shen H., Cao Y., Zhang J., Cai Y., Wang R., Jiang Y. Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans. Int. J. Antimicrob. Agents. 2009;33:258–263. doi: 10.1016/j.ijantimicag.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Parraga P., Sanchez-Fresneda R., Zaragoza O., Arguelles J.C. Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochim. Biophys. Acta. 2011;1810:777–783. doi: 10.1016/j.bbagen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto Y., Aoki S., Mataga I. Enhancement of amphotericin B activity against Candida albicans by superoxide radical. Mycopathologia. 2004;158:9–15. doi: 10.1023/B:MYCO.0000038430.20669.80. [DOI] [PubMed] [Google Scholar]
- 34.Fernandes L., Rodrigues-Pousada C., Struhl K. Yap, a novel family of eight bZIP proteins in Saccharomyces cerevisiae with distinct biological functions. Mol. Cell. Biol. 1997;17:6982–6993. doi: 10.1128/mcb.17.12.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J., Godon C., Lagniel G., Spector D., Garin J., Labarre J., Toledano M.B. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 1999;274:16040–16046. doi: 10.1074/jbc.274.23.16040. [DOI] [PubMed] [Google Scholar]
- 36.Hughes L.M., Lanteri C.A., O’Neil M.T., Johnson J.D., Gribble G.W., Trumpower B.L. Design of anti-parasitic and anti-fungal hydroxy-naphthoquinones that are less susceptible to drug resistance. Mol. Biochem. Parasitol. 2011;177:12–19. doi: 10.1016/j.molbiopara.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clinical and Laboratory Standards Institute (CLSI) Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi: Approved Standard—Second edition. CLSI document M38-A2. Vol. 28 Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2008. [Google Scholar]
- 38.Odds F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 39.Kirkman T.W. Statistics to use. [(accessed on 10 December 2012)]. Available online: http://www.physics.csbsju.edu/stats/
- 40.Vincent J.M. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;159:850. doi: 10.1038/159850b0. [DOI] [PubMed] [Google Scholar]
- 41.Kim J., Campbell B., Mahoney N., Chan K., Molyneux R., May G. Chemosensitization prevents tolerance of Aspergillus fumigatus to antimycotic drugs. Biochem. Biophys. Res. Commun. 2008;372:266–271. doi: 10.1016/j.bbrc.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 42.Burnett C.L., Bergfeld W.F., Belsito D.V., Hill R.A., Klaassen C.D., Liebler D.C., Marks J.G., Jr., Shank R.C., Slaga T.J., Snyder P.W., et al. Final report of the safety assessment of kojic acid as used in cosmetics. Int. J. Toxicol. 2010;29:244S–273S. doi: 10.1177/1091581810385956. [DOI] [PubMed] [Google Scholar]