Table 2.
Linear correlation equation between ΔacidG°(CH) or ΔacidG°(OH) and σp−(g), σp−(aq) or 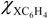 for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
| Y | Δacid G° | b | M | R2 |
|---|---|---|---|---|
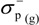 |
CH | 344.9 | −23.0 | 0.99 |
| (347.1) | (−25.2) | (0.98) | ||
| OH | 339.2 | −9.7 | 0.96 | |
| (353.3) | (−1.0) | (0.97) | ||
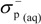 |
CH | 345.6 | −20.1 | 0.97 |
| (343.6) | (−18.2) | (0.98) | ||
| OH | 338.9 | −7.7 | 0.95 | |
| (352.7) | (−8.3) | (0.95) | ||
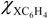 |
CH | 472.5 | −24.7 | 0.98 |
| (488.0) | (−27.3) | (0.97) | ||
| OH | 393.3 | −10.4 | 0.98 | |
| (409.2) | (−10.8) | (0.99) |
