Table 1.
Structure of the discussed ring-substituted 2-hydroxynaphthalene-1-carboxanilides 1–8c, experimentally determined values of lipophilicity log k, predicted electronic Hammett’s σ parameters, and IC50 values related to PET inhibition in spinach chloroplasts in comparison with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) standard, in vitro antibacterial activity (MIC) of compounds in comparison with ampicillin (APC) standard; in vitro antimycobacterial activity (MIC) of compounds in comparison with isoniazid (INH) standard, and in vitro cytotoxicity assay (LD50) of choice compounds.
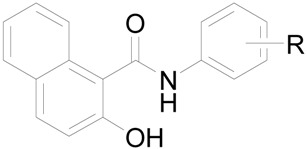 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R | log k | σa | [µmol/L] | |||||||||
| PET IC50 | MIC | LD50 | |||||||||||
| SA | MRSA 63718 | MRSA 630 | MRSA 3202 | MM | MK | MS | MAP | ||||||
| 1 | H | 1.3016 | 0 | 28.9 | >972 | >972 | 243 | 122 | 60.7 | 15.2 | 486 | 950 | >20 |
| 2a | 2-OCH3 | 0.5121 | −0.28 | 477 | >873 | >873 | >873 | >873 | >873 | >873 | >873 | 852 | – |
| 2b | 3-OCH3 | 0.6582 | 0.12 | 681 | 436 | 436 | 873 | 218 | 218 | 109 | 218 | 426 | – |
| 2c | 4-OCH3 | 0.6342 | −0.27 | ND | >873 | >873 | >873 | >873 | 109 | 218 | 436 | 852 | – |
| 3a | 2-CH3 | 0.7962 | −0.17 | 586 | 462 | 462 | 462 | 462 | 231 | 115 | 462 | 451 | – |
| 3b | 3-CH3 | 0.4593 | −0.07 | 372 | 231 | 462 | 231 | 231 | 231 | 115 | 231 | 451 | – |
| 3c | 4-CH3 | 0.3751 | −0.17 | 874 | 231 | 462 | 462 | 462 | 115 | 115 | >923 | 451 | – |
| 4a | 2-F | 0.5664 | 0.06 | 243 | 228 | 455 | 455 | 455 | 228 | 114 | 228 | 203 | – |
| 4b | 3-F | 0.5025 | 0.34 | 213 | 228 | >910 | >910 | 228 | >910 | 114 | >910 | >889 | – |
| 4c | 4-F | 0.5568 | 0.06 | 313 | >910 | >910 | >910 | >910 | >910 | >910 | >910 | >889 | – |
| 5a | 2-Cl | 0.8984 | 0.22 | 49.6 | 215 | 860 | >860 | >860 | 107 | 107 | 107 | 202 | >20 |
| 5b | 3-Cl | 0.7904 | 0.37 | 79.7 | >860 | >860 | >860 | 107 | >860 | 53.7 | 215 | 840 | – |
| 5c | 4-Cl | 0.7908 | 0.23 | 59.2 | 215 | >860 | >860 | 107 | >860 | 53.7 | >860 | 420 | – |
| 6a | 2-Br | 0.9509 | 0.22 | 52.2 | >748 | >748 | >748 | >748 | >748 | 93.5 | >748 | 731 | – |
| 6b | 3-Br | 0.8595 | 0.39 | 61.1 | >748 | >748 | >748 | >748 | >748 | 46.7 | >748 | 731 | – |
| 6c | 4-Br | 0.8790 | 0.23 | 102 | 47.0 | 187 | 47.0 | 94.1 | 187 | 93.5 | 187 | 175 | 8.0 |
| 7a | 2-CF3 | 0.8353 | 0.51 | 153 | 97.1 | 193 | 97.1 | 193 | 96.6 | 96.6 | 386 | 377 | >20 |
| 7b | 3-CF3 | 0.9411 | 0.43 | 45.6 | >748 | 187 | 374 | 94 | >748 | 93.5 | >748 | 731 | >20 |
| 7c | 4-CF3 | 0.9994 | 0.51 | 29.0 | >748 | 94 | 94 | 47 | >748 | 23.3 | >748 | 731 | 3.3 |
| 8a | 2-NO2 | 0.8501 | 0.77 | 121 | 26.0 | 415 | 104 | 52 | 104 | 51.9 | 208 | 195 | >20 |
| 8b | 3-NO2 | 0.9187 | 0.71 | 86.4 | 208 | 26.0 | 208 | 208 | 51.9 | 104 | 208 | 405 | >20 |
| 8c | 4-NO2 | 0.6260 | 0.78 | 37.5 | >830 | 830 | 415 | 104 | 51.9 | 415 | 208 | 811 | 2.5 |
| DCMU | – | 0.8801 | 0.6 | 1.9 | – | – | – | – | – | – | – | – | – |
| APC | – | 0.4337 | – | – | 5.7 | >45.8 | >45.8 | >45.8 | – | – | – | – | – |
| INH | – | 0.0141 | – | – | – | – | – | – | 467 | 29.2 | 117 | >1823 | – |
a calculated using ACD/Percepta ver. 2012 (Advanced Chemistry Development, Inc., Toronto, ON, Canada, 2012); SA = Staphylococcus aureus ATCC 29213, MRSA = clinical isolates of methicillin-resistant Staphylococcus aureus 63718, SA 630 and SA 3202 (National Institute of Public Health, Prague, Czech Republic); MM = M. marinum CAMP 5644, MK = M. kansasii DSM 44162, MM = M. smegmatis ATCC 700084 and clinical isolate MAP = M. avium paratuberculosis CIT03; ND = not determined due to its interaction with 2,6-dichlorophenol-indophenol (DCPIP).
